Hi CMS,
The anode electrode should be accepting most of the electrons, however just in case there is a side fermentation, it would not hurt to connect tubing that will allow gas to escape from your MFC.
Donna
Soil-Based Microbial Fuel Cells
Moderators: AmyCowen, kgudger, MadelineB, Moderators
-
donnahardy2
- Former Expert
- Posts: 2671
- Joined: Mon Nov 14, 2005 12:45 pm
-
Crazy_Mad_Scientist
- Posts: 71
- Joined: Wed Sep 14, 2016 7:14 pm
- Occupation: Student
Re: Soil-Based Microbial Fuel Cells
Hi Donna,
I was looking at this MFC diagram to better understand some of the chemical reactions occuring in the cell. I don't quite understand what the H+ is. I used to always think that they were hydrogen ions, which would just leave the anode as hydrogen gas in the cathode (since Pb2+ would not combine with it to form water). However doing some further research makes me think that the H+ would combine with Pb2+. What would really happen when H+ combines with Pb2+? Here is the picture for reference.
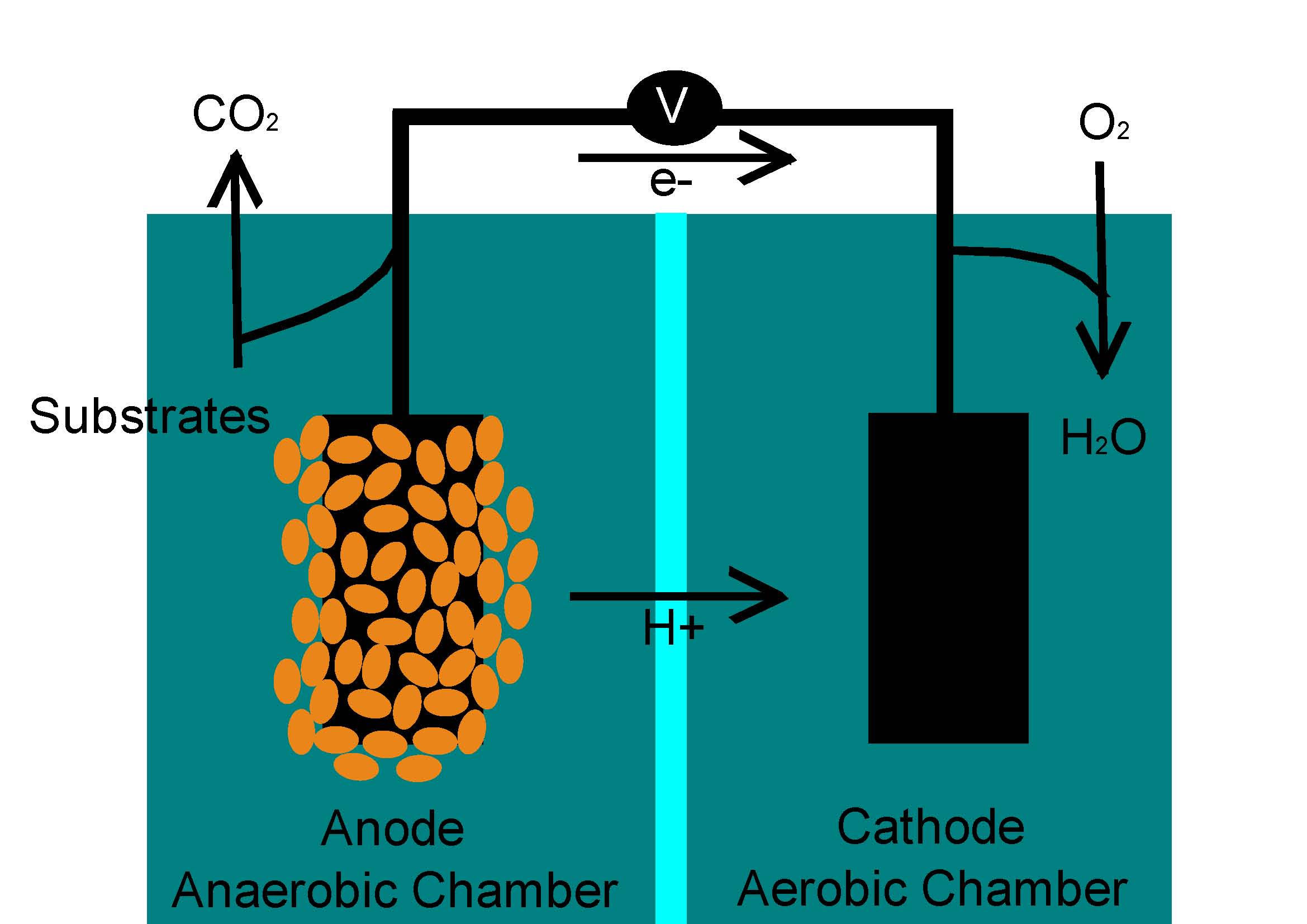
I think I confused the microbial electrolysis cell (MEC) with the MFC. Apparently an additional voltage is supplied to reduce the protons into hydrogen gas. If this is true I will probably attach a tube in the anode chamber to get rid of the excess methane gas? I think that this can be applicable to commercial MFCs in the future (to harness the methane gas produced from MFCs to be used in internal combustion engines in order to replace fossil fuels?)
Thank you!
CMS
I was looking at this MFC diagram to better understand some of the chemical reactions occuring in the cell. I don't quite understand what the H+ is. I used to always think that they were hydrogen ions, which would just leave the anode as hydrogen gas in the cathode (since Pb2+ would not combine with it to form water). However doing some further research makes me think that the H+ would combine with Pb2+. What would really happen when H+ combines with Pb2+? Here is the picture for reference.

I think I confused the microbial electrolysis cell (MEC) with the MFC. Apparently an additional voltage is supplied to reduce the protons into hydrogen gas. If this is true I will probably attach a tube in the anode chamber to get rid of the excess methane gas? I think that this can be applicable to commercial MFCs in the future (to harness the methane gas produced from MFCs to be used in internal combustion engines in order to replace fossil fuels?)
Thank you!
CMS
-
Crazy_Mad_Scientist
- Posts: 71
- Joined: Wed Sep 14, 2016 7:14 pm
- Occupation: Student
Re: Soil-Based Microbial Fuel Cells
By the way, is it possible to post the endorsement for Experiment? Thank you so much! 
-
donnahardy2
- Former Expert
- Posts: 2671
- Joined: Mon Nov 14, 2005 12:45 pm
Re: Soil-Based Microbial Fuel Cells
Hi CMS,
I'm so sorry for the delay. I have been having trouble logging on to my new computer; hopefully the issues are resolved now.
You are correct; the magnesium sulfate is a non-toxic electrolyte that is completely ionized when in solution, so it is highly conductive. The acetic acid is a weakly ionized molecule, so is not as conductive. Chloride is corrosive and it is possible for the chloride ions to be oxidized to chlorine, which is a toxic gas. The science buddies project guide information is correct.
Have you set up your MFC yet? Please let me know if you are making progress.
I will try again to complete the endorsement.
Donna
I'm so sorry for the delay. I have been having trouble logging on to my new computer; hopefully the issues are resolved now.
You are correct; the magnesium sulfate is a non-toxic electrolyte that is completely ionized when in solution, so it is highly conductive. The acetic acid is a weakly ionized molecule, so is not as conductive. Chloride is corrosive and it is possible for the chloride ions to be oxidized to chlorine, which is a toxic gas. The science buddies project guide information is correct.
Have you set up your MFC yet? Please let me know if you are making progress.
I will try again to complete the endorsement.
Donna
-
Crazy_Mad_Scientist
- Posts: 71
- Joined: Wed Sep 14, 2016 7:14 pm
- Occupation: Student
Re: Soil-Based Microbial Fuel Cells
Hi Donna,
I set up my MFC yesterday, and the voltage spiked up to 480 mV. However in the morning when I checked the cell, the voltage was around 120 mV. In addition the cathode chamber turned quite yellow. Do you know what could have caused this problem? I searched everywhere but I can't find a solution.
Thank you!
CMS
I set up my MFC yesterday, and the voltage spiked up to 480 mV. However in the morning when I checked the cell, the voltage was around 120 mV. In addition the cathode chamber turned quite yellow. Do you know what could have caused this problem? I searched everywhere but I can't find a solution.
Thank you!
CMS
-
Crazy_Mad_Scientist
- Posts: 71
- Joined: Wed Sep 14, 2016 7:14 pm
- Occupation: Student
Re: Soil-Based Microbial Fuel Cells
Hi Donna,
I'm not sure if you will read this reply, but I just wanted to update you on what has happenned in my experiment .
.
So first of all, I did not end up using Pb2+ as the electron acceptors, as I was just too concerned about toxicity issues (I'm doing the experiment at home). Instead, I used Copper sulfate pentahydrate (Cu2+) for the test run. I am currently using several metal ions (zinc, nickel and copper) to precipitate several metals using different potential differences.
I am extremely happy with the Voltages I was able to generate! I ended up using some garden soil for the test run, but was still able to consistently generate aroun 612 mV! The resistance reduce from 1300 to about 780. Thus the Cu2+ was precipitated onto the electrode in less than a day. (I used freezing point and density measurements to screen for the precipitation of copper). The cool thing was that the specific gravity decreased below the original electrolyte solution, showing that not only copper was precipitated onto the cathode. This could mean that the magnesium sulfate or sugar in the solution was also removed from the solution. In the future I will probably create a copper hydroxide solution to determine the presence and amount of copper in the solution. I'll just weigh the dried precipitate.
Currently, I am developing this topic by using yeast in the anode and waste paper as the feed stock. (I will apply a pretreatment onto the cellulose and further add sulfuric acid to hydrolze the paper into glucose. This will allow me to create bioethanol as well.
This project is really turning into an innovation, because I am combining several known and well researched fields together. (Generating electricity from waste paper, reducing metals, and producing bioethanol). I decided to work on this topic since MFCs actually have so much more potential than just generating electricity. I'm also pretty sure that waste paper was never considered for use in MFCs before.
For waste paper, I will:
a) Apply oxdiative delignification pretreatment onto some paper to allow access to the cellulose (I chose this method because I can just use some hydrogen peroxide). I will also create a 1% NaOH solution.
b) Hydrolyse the paper for several hours in an incubator using either 1 M sulfuric acid or HCl depending on what's available in school.
c) Measure glucose content using glucose test strips or convert specific gravity measurements.
For metal reduction, I will:
a) Create a 1 g/L dilution of Cu2+, Ni2+, Zn2+, and maybe some other metals in the cathode.
b) Vary the Voltage potential by adding different concentrations of nutrients (explained later) to recover the different metals.
c) Take measurements of metal contents using sodium hydroxide and weighing the precipitate.
For bioethanol, I will:
a) Create a mixed culture of bakers yeast and probiotic yeast strain; add beef stock, vitamin powder, and nutrients
b) Add glucose from waste paper, and feed the MFC using an aquarium tube.
c) Take hydrometer readings to calculate ethanol produced; take conductivity measurements and additional measurements (freezing point, etc).
Thank you so much for helping with my project! Your feedback on my new project would be highly appreciated! I'll definately keep you updated on any questions I have on my project.
CMS
I'm not sure if you will read this reply, but I just wanted to update you on what has happenned in my experiment
So first of all, I did not end up using Pb2+ as the electron acceptors, as I was just too concerned about toxicity issues (I'm doing the experiment at home). Instead, I used Copper sulfate pentahydrate (Cu2+) for the test run. I am currently using several metal ions (zinc, nickel and copper) to precipitate several metals using different potential differences.
I am extremely happy with the Voltages I was able to generate! I ended up using some garden soil for the test run, but was still able to consistently generate aroun 612 mV! The resistance reduce from 1300 to about 780. Thus the Cu2+ was precipitated onto the electrode in less than a day. (I used freezing point and density measurements to screen for the precipitation of copper). The cool thing was that the specific gravity decreased below the original electrolyte solution, showing that not only copper was precipitated onto the cathode. This could mean that the magnesium sulfate or sugar in the solution was also removed from the solution. In the future I will probably create a copper hydroxide solution to determine the presence and amount of copper in the solution. I'll just weigh the dried precipitate.
Currently, I am developing this topic by using yeast in the anode and waste paper as the feed stock. (I will apply a pretreatment onto the cellulose and further add sulfuric acid to hydrolze the paper into glucose. This will allow me to create bioethanol as well.
This project is really turning into an innovation, because I am combining several known and well researched fields together. (Generating electricity from waste paper, reducing metals, and producing bioethanol). I decided to work on this topic since MFCs actually have so much more potential than just generating electricity. I'm also pretty sure that waste paper was never considered for use in MFCs before.
For waste paper, I will:
a) Apply oxdiative delignification pretreatment onto some paper to allow access to the cellulose (I chose this method because I can just use some hydrogen peroxide). I will also create a 1% NaOH solution.
b) Hydrolyse the paper for several hours in an incubator using either 1 M sulfuric acid or HCl depending on what's available in school.
c) Measure glucose content using glucose test strips or convert specific gravity measurements.
For metal reduction, I will:
a) Create a 1 g/L dilution of Cu2+, Ni2+, Zn2+, and maybe some other metals in the cathode.
b) Vary the Voltage potential by adding different concentrations of nutrients (explained later) to recover the different metals.
c) Take measurements of metal contents using sodium hydroxide and weighing the precipitate.
For bioethanol, I will:
a) Create a mixed culture of bakers yeast and probiotic yeast strain; add beef stock, vitamin powder, and nutrients
b) Add glucose from waste paper, and feed the MFC using an aquarium tube.
c) Take hydrometer readings to calculate ethanol produced; take conductivity measurements and additional measurements (freezing point, etc).
Thank you so much for helping with my project! Your feedback on my new project would be highly appreciated! I'll definately keep you updated on any questions I have on my project.
CMS

