Abstract
Have you ever wondered how antibiotics and other medicines are able to stop dangerous infections? How do such medications kill microorganisms without in general harming the person the microorganisms are infecting? Because many different types of microorganisms can infect us, we have had to develop an amazing number of ways to deal with these harmful microbes. Fungal infections can be particularly dangerous, but we have developed many different antifungal medications that can usually deal with these infections. But how do antifungal medications work, and how effective are they? In this science project, you will test how well different common antifungal medications can stop the growth of baker's yeast, a harmless variety of fungus often used in baking.Summary
Edited by Svenja Lohner, PhD, Science Buddies
Recommended Project Supplies
Objective
Determine how different antifungal medications slow or stop the growth of fungus.
Introduction
Left untreated, fungal infections can lead to serious medical conditions. Consequently, it is important to know what kind of antifungal medicine, and how much, to take to kill a certain type of fungus. Some of the most common fungal infections are athlete's foot, nail infections, and yeast infections. The fungus group includes molds, yeast, mushrooms, and more. Fungi actually make up a kingdom of organisms separate from plants, animals, and bacteria.
Different antifungal agents work in different ways to kill fungus. Two of the most common antifungal agents used in nonprescription antifungal medicines are azoles and allylamines. Azole and allylamine both work by disrupting the fungus' ability to make ergosterol, which is a chemical compound important for the fungus to make a strong cell membrane. Without a strong cell membrane, the fungal cells could become leaky and die. Ergosterol is not in plant or animal cells, which makes it a good compound to target if you only want to kill the fungus without hurting infected plants or animals (including people).
Azoles and allylamines disrupt the fungus' ability to make ergosterol in different ways, as shown in the molecular pathway diagram in Figure 1. A molecular pathway is a series of chemical reactions that take place within a cell. In this diagram, arrows pointing down indicate a chemical reaction. This means that squalene is normally converted to lanosterol during a chemical reaction aided by the enzyme squalene epoxidase. An enzyme is something that helps a chemical reaction take place. The lanosterol is then converted to ergosterol with the help of the enzyme lanosterol 14α-demethylase. Ergosterol is used by the fungal cells to create a functional cell membrane. The sideways "T"s in the diagram show how a chemical reaction can be stopped. Allylamines stop the function of the squalene epoxidase enzyme, thus blocking the fungus from making lanosterol. Azoles stop the function of the enzyme lanosterol 14α-demethylase, thus blocking the fungus from making ergosterol.
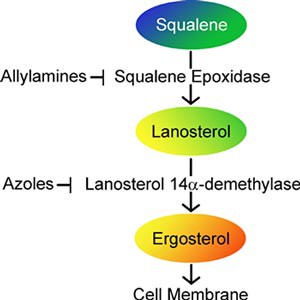 Image Credit: Teisha Rowland, Science Buddies / Science Buddies
Image Credit: Teisha Rowland, Science Buddies / Science BuddiesThe diagram shows two antifungal agents, azoles and allylamines that disrupt the same molecular pathway, but at different points. This pathway starts with squalene which is converted to lanosterol with an enzyme called squalene epoxidase, this enzyme is blocked by allylamines. Lanosterol is converted to ergosterol with an enzyme called lanosterol 14α-demethylase, this enzyme is blocked by azoles. Ergosterol is needed for the growth of cell membranes in fungus.
Figure 1. Two groups of common antifungal agents, azoles and allylamines, work in different ways to disrupt the fungus' ability to make ergosterol. Fungus needs ergosterol to make strong cell membranes.
A common nonprescription azole found in antifungal medications is called clotrimazole, while a common nonprescription allylamine is called terbinafine. Tolnaftate (also sold as Tinactin) is another common nonprescription antifungal agent, and it is thought to inhibit squalene epoxidase, like the allylamines. Nonprescription medicines, also called "over-the-counter" medicines, do not require a doctor's prescription to buy, so that anyone can go into a pharmacy and buy them, while a doctor's prescription is needed to buy prescription medicines.
Undecylenic acid, another common nonprescription antifungal agent, can be used to fight fungal infections as well as infections caused by other microorganisms. Undecylenic acid is derived from the castor bean, which contains toxic compounds. Scientists do not completely understand how undecylenic acid fights fungal infections, but they think that it interferes with the fungus' life cycle. Specifically, it may stop the fungus from reproducing (making more fungus).
Different types of fungus react to antifungal agents in different ways, and fungus can even become resistant to certain antifungals agents. For example, if a fungus starts making a large amount of the enzyme lanosterol 14α-demethylase, it may become resistant to azole because no matter how much of the azole is used, the fungus could still have enough lanosterol 14α-demethylase to be able to turn lanosterol to ergosterol.
In this science project, you will investigate how efficient different antifungal agents are at stopping fungus from growing. Baker's yeast is a type of fungus used in baking and can easily be grown at home. As yeasts grow and reproduce, they make carbon dioxide, which is what makes bread rise. Which antifungal agents are most efficient at stopping yeast from growing? What does this say about the relative importance of the mechanisms that these antifungal agents disrupt in the yeast?
Terms and Concepts
- Fungal infections
- Fungus
- Antifungal
- Azoles
- Allylamines
- Ergosterol
- Molecular pathway
- Lanosterol
- Undecylenic acid
- Resistance
Questions
- How is ergosterol related to both allylamine and azole antifungal agents?
- How do allylamine and azole antifungal agents work differently to stop fungal infections?
- What is the name of a common azole found in antifungal medicines? What about a common allylamine?
- How do scientists think undecylenic acid stops fungal infections?
- How might you be able to measure whether yeast is growing? Hint: Think about what yeast produces that makes bread rise.
- Which type of antifungal agents (allylamines, azoles, or undecylenic acid) do you think will be most effective at stopping yeast from growing? Why, based on their different molecular mechanisms, do you think this is so?
Bibliography
These resources are a good place to start gathering information about fungal infections and antifungal medications:
- U.S. National Library of Medicine, National Institutes of Health: Bookshelf. (1996). Chapter 76 antifungal agents. Retrieved September 28, 2011.
- Drugs.com. (n.d.). Azole antifungals. Retrieved September 28, 2011.
- Thorne Research, Inc. (2002, February). Undecylenic acid. Retrieved October 29, 2013.
The range of antifungal agent concentrations suggested for this science project are based on these scientific studies/reports:
- Sud, I., and D. S, Feingold. (1981, July). Heterogeneity of action mechanisms among antimycotic imidazoles. Antimicrobial Agents and Chemotherapy. Published online. Retrieved October 4, 2011.
- Tiballi, R. N. et al. (1995, December). Saccharomyces cerevisiae infections and antifungal susceptibility studies by colorimetric and broth macrodilution methods. Diagnostic Microbiology and Infectious Disease. Published online. Retrieved October 4, 2011.
- University of Adelaide: Mycology Online. (2011). Antifungal susceptibility testing. Retrieved October 4, 2011.
For tips on growing microorganisms such as yeast, see this Science Buddies resource:
- Science Buddies. (1999). Why won't my cultures grow?. Retrieved September 28, 2011.
Materials and Equipment 
Recommended Project Supplies
- Measuring Gas Production Kit, available from our partner
Home Science Tools.
You will need these items from the kit:
- 250-mL graduated cylinder
- 100-mL graduated cylinder
- Wide-mouth, 8 oz. squirt bottles (4)
- Clear plastic tubing
- Waterproof thermometer
- You will also need to gather these items, not included in the kit:
- Dry yeast. Tip: Buying a whole jar is probably more economical than individual packets.
- Disposable gloves. Can be purchased at a local drug store or pharmacy. If you are allergic to latex, use vinyl or polyethylene gloves.
- Plastic tub or bucket
- Water
- Optional: Plastic wrap
- Packing tape
- Permanent marker
- Measuring spoons
- Sugar
- Measuring cup
- Warm water, typically 43-46°C (about 110°F–115°F), but consult the recommendations on your yeast package
- Clock or timer
- Different antifungal medicines (3), each using a different type of antifungal agent. If it is not labeled on the front of the packaging, look under the "active ingredient" section on the back of the package to see what antifungal agent is being used in the medicine. Antifungal agents commonly found in nonprescription antifungal medicines are clotrimazole, terbinafine, tolnaftate, and undecylenic acid.
- Optional: Toothpicks
- Optional: Fork
- Graph paper
- Lab notebook
Disclaimer: Science Buddies participates in affiliate programs with Home Science Tools, Amazon.com, Carolina Biological, and Jameco Electronics. Proceeds from the affiliate programs help support Science Buddies, a 501(c)(3) public charity, and keep our resources free for everyone. Our top priority is student learning. If you have any comments (positive or negative) related to purchases you've made for science projects from recommendations on our site, please let us know. Write to us at scibuddy@sciencebuddies.org.
Experimental Procedure
For health and safety reasons, science fairs regulate what kinds of biological materials can be used in science fair projects. You should check with your science fair's Scientific Review Committee before starting this experiment to make sure your science fair project complies with all local rules. Many science fairs follow Regeneron International Science and Engineering Fair (ISEF) regulations. For more information, visit these Science Buddies pages: Project Involving Potentially Hazardous Biological Agents and Scientific Review Committee. You can also visit the webpage ISEF Rules & Guidelines directly.
Before you start the experiment, read about the antifungal agents you picked to test and find out how they stop fungal infections.
Setting up the Gas Collection Apparatus
In the first part of this procedure, you will be setting up the gas collection apparatus. In the process of growing, yeast produce carbon dioxide (CO2), and if the number of living yeast decreases, the amount of CO2 produced will also decrease. Consequently, measuring CO2 production is a good way to determine how much yeast is alive relative to the other conditions you test. The gas collection apparatus that you set up here will show you how much CO2 is being produced by the yeast.
- Remove the small red cap from one of the squeeze bottles. Then connect the tubing to the tip opening, as shown in Figure 2. Make sure that you have a tight fit.
 Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Figure 2. Tube connected to the bottle opening.
- You will be collecting carbon dioxide from the yeast by displacing water trapped in an inverted graduated cylinder. Here's how to set it up:
- Fill your plastic dishpan (or bucket) about one-third full with water.
- Fill the 100-mL graduated cylinder with water.
- If your dishpan is deep enough, fill the graduated cylinder by tipping it on its side inside the dishpan. Allow any bubbles to escape by tilting the cylinder up slightly, while keeping it under water. Keeping the opening of the cylinder under water, turn it upside down and attach it to the side of the dishpan with packing tape (or have your helper hold it in place).
- If your dishpan is not deep enough, fill the graduated cylinder completely using the faucet and cover the top tightly with plastic wrap. Quickly invert the cylinder and place the opening in the dishpan, beneath the surface of the water. Remove the plastic wrap. Attach the cylinder to the side of the tub with packing tape (or have your helper hold it in place).
-
The graduated cylinder should now be upside down, full of water and with its opening under the surface of the water in the dishpan. Place the free end of the tubing from the plastic bottle inside the graduated cylinder. Your apparatus is now ready to trap carbon dioxide from the yeast (see Figure 3).
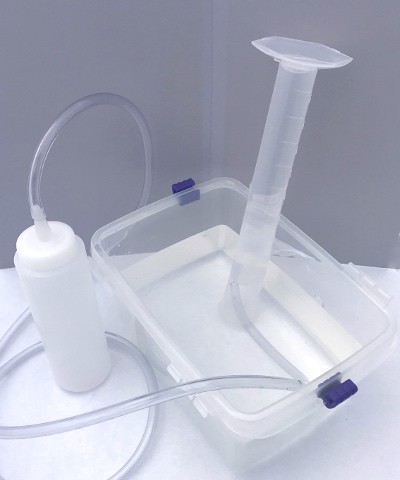 Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Image Credit: Svenja Lohner, Science Buddies / Science BuddiesA graduated cylinder is placed upside-down in a tub of water and a plastic tube enters the cylinder from underwater. The plastic tube is connected to the nozzle of a squeeze bottle. Air from the squeeze bottle will be funneled into the upside-down cylinder and will be trapped by the water below.
Figure 3. Picture of the inverted graduated cylinder gas collection apparatus.
- You can test your gas collection apparatus by removing the tube from the bottle top and blowing gently into the tube. The bubbles you create should be captured inside the cylinder. (You will need to reconnect the tube to the bottle and re-fill the cylinder before starting your experiment.)
Testing Different Antifungal Medicines
In the second part of this procedure, you will be testing how effective different antifungal agents are on yeast. After following the steps in the "Setting up the Gas Collection Apparatus" section, you will be ready to test different antifungal agents. First you will grow yeast and collect the CO2 produced without adding any antifungal agents. This will be your control. Then you will test two different concentrations of each antifungal agent.
- Using the permanent marker, label each of the bottles with the name of the antifungal agent you will be testing, along with the concentration that you will test. Label an extra bottle "control." When changing the concentration for one antifungal agent, you can re-use the bottle that you have used for the same antifungal agent before. Just make sure to start with the lowest concentration first, and rinse the bottle thoroughly between experiments.
- Dissolve 1 teaspoon (tsp.) of sugar in ½ cup of warm water (110°F to 115°F, or whatever temperature the yeast package recommends). When the sugar is fully dissolved, add ½ tsp. of yeast, mix well, and pour into the appropriate bottle. Tip: It may help to use a fork to mix in the yeast. Be sure to note the actual temperature of the water in your lab notebook.
- Cap the bottle tightly using the cap with the tubing attached. Make sure the open end of the collection tube is still inside the submerged gas-collecting graduated cylinder. Note the starting time in your lab notebook.
- There should be water in the tubing as soon as it is submerged in the water. The CO2 gas will push some water out of the tubing before the graduated cylinder starts to fill with CO2 gas.
- Within five to ten minutes, the yeast should start foaming, and soon you should see bubbles collecting in the graduated cylinder. Note the time when you first start seeing bubbles in your lab notebook.
- Decide how long to collect CO2 (somewhere between 15–30 minutes is probably good, but you may need to adjust for your particular conditions). Use the same amount of time for all of your tests.
- Note: Do not let the graduated cylinder become completely filled with CO2, but instead stop it before this point. If you let it become completely filled, and the next condition you test makes even more CO2, this could lead to poor and inaccurate results because your graduated cylinder may fill up before your test time is over.
- Tip: If your solution makes a large amount of CO2 very quickly, you can try to make it produce less CO2 by using less sugar and possibly less yeast. For example, you could repeat this step using ½ tsp. sugar (instead of 1 tsp.) and ¼ tsp. yeast (instead of ½ tsp.).
- When the time is up, note how much CO2 was collected by observing how much water was displaced from the graduated cylinder.
- Re-fill your gas collection cylinder, and carefully rinse out the yeast solution from the bottle. Repeat steps 2–6. You should run at least three separate trials for each testing condition.
- Next, prepare your antifungal test solutions. You will be testing each antifungal agent at two different concentrations, a low concentration and a high concentration. The concentration range that you are testing is based on concentrations used in scientific studies listed in the Bibliography. You will be testing each antifungal agent and concentration one at a time (unless you decide to set up more than one gas collection apparatus).
- First, check the "active ingredient" box on the antifungal medication's packaging and note what percentage of antifungal agent is in the medication. If you are using an antifungal medicine that has a concentration of antifungal agent greater than 1%, you will need first to dilute the medicine so that it is at a 1% concentration.
- For example, if the antifungal medicine is made up of 25% of antifungal agent, you will need to make a 25-fold dilution. You can do this by mixing 1/8 tsp. of the antifungal agent with 3 tsp. of warm water.
- Start by preparing the high test concentration of the antifungal medicine. Take your medicine (or dilution) with 1% active ingredient (antifungal agent) and dilute it using the following steps to generate your high test concentration.
- First, dilute your antifungal medicine tenfold. To do this, measure 1/8 tsp. of antifungal medicine and mix this with 9/8 tsp. water (that is, 1 tsp. plus another 1/8 tsp.). Make sure you get all of the antifungal medicine off the measuring spoon. Using a toothpick may help. (Remember to wear latex gloves to protect your hands.)
- Mix this tenfold dilution very well. You may want to use a fork to vigorously stir, or whisk, the dilution. Mix it until almost all of the tiny pieces of antifungal medicine are not visible (although there may still be a small number visible).
- Next, measure ¼ tsp. of this tenfold dilution and add it to the ½ cup solution of warm water and sugar (as made in step 2, without the yeast). Mix well. What is the final-fold dilution of antifungal agent that you are using?
- Because you performed a tenfold dilution in step 10a, and a 100-fold dilution in step 10c, you will be using a 1000-fold dilution for these tests. See the Technical Note for calculations used to determine the concentration of the antifungal solution you just made.
Technical NoteBy following steps 10a to 10c, you created a 1:1000 dilution of the antifungal agent, or an antifungal solution that is at a concentration of 10 µg/mL. This will be your high test concentration in your test and can be calculated by doing the following measurement conversions and calculations.
In step 10a, you used 1/8 tsp. of antifungal medicine. The conversions below can be used to find out what 1/8 tsp. equals in grams (g).
Measurement conversions:
We make the assumption that water and the antifungal medicine have similar weights. This is reasonable, because the medicine contains a lot of water.
This means that 1/8 tsp. of antifungal medicine weighs approximately 0.62 g. Consequently, you used 0.62 g of antifungal medicine in step 10a. This was in a total volume of 10/8 tsp., so in step 10c when you used ¼ tsp., you were using 1/5 of the 0.62 g, which is 0.124 g. 0.124 grams equals 124 milligrams (mg).
Because the medicine has the antifungal agent at a concentration of 1%, this means that the 124 mg of medicine you used only has 1% antifungal agent, or 1.24 mg antifungal agent. 1.24 mg equals 1240 micrograms (µg).
In step 10c, this 1240 µg antifungal agent was diluted into ½ cup water, and ½ cup equals 120 mL. If you divide 1240 µg by 120 mL, you get about 10 µg/mL, the final concentration of antifungal agent in the high test solution.
- Next, make your low test concentration of the antifungal medicine.
- Use the 1000-fold dilution you prepared in the previous step.
- Measure out ¼ tsp. of the 1000-fold dilution and add it to another ½ cup solution of warm water and sugar (as made in step 2, without the yeast). Mix well. What is the final-fold dilution of antifungal agent that you are using? What is the final low test concentration of antifungal agent?
- When you are done preparing both of your test solutions, start running the antifungal agent experiments.
- Start with the low test concentration experiment. Take the warm sugar solution with the low test concentration of antifungal agent and add ½ tsp. of yeast, mix well, and pour into the appropriate bottle. If the solution has cooled down too much, carefully heat it up to the right temperature again. Make sure not to heat it too much as this might affect the antifungal agent! Note the temperature in your notebook.
- Repeat steps 3 to 7, to test the antifungal agents.
- In step 4, you may not see foaming or the formation of bubbles. Why do you think this happens? Note in your lab notebook whether you see foaming or bubbling for each condition tested.
- Once you have completed the low test concentration trials, rinse your bottle thoroughly and start the high test concentration experiments.
- Add ½ tsp. of yeast to the high test concentration solution, mix well, and pour into the appropriate bottle.
- Then repeat steps 3–7 again. If the solution has cooled down too much, carefully heat it up to the right temperature again before you start your experiment. Make sure not to heat it too much as this might affect the antifungal agent! Note the temperature in your notebook.
- Run at least three separate trials for each antifungal agent concentration tested, and three separate trials of the yeast without any antifungal medicine added.
- Make sure to use the same water temperature each time you make a solution, because yeast activity is temperature dependent.
- Multiple trials help scientists make sure that their results are accurate and reproducible.
Analyzing Your Data
- Calculate the average volume of the CO2 produced for each amount of antifungal agent tested and write this in your lab notebook.
- Make a graph of your results.
- Write the different antifungal medicines and concentrations tested on the x-axis (the horizontal axis).
- Plot the corresponding average volume of CO2 produced on the y-axis (the vertical axis).
- Which antifungal medicine was most effective at stopping the yeast from growing? Did any completely stop antifungal growth? Which was least effective? How do you justify your reasoning? Why do you think one worked better than another? Did any of the antifungal medicines have the same effect at both the higher and lower concentrations tested?
Ask an Expert
Global Connections
The United Nations Sustainable Development Goals (UNSDGs) are a blueprint to achieve a better and more sustainable future for all.
Variations
- There are many substances and essential oils that are thought to have some antifungal properties, such as tea tree oil, zinc (a dietary supplement), citronella oil, and several others. Read more about these other antifungal substances and use this experimental procedure as the basis to test which of these other antifungal substances are most effective. Are any of them more effective at stopping yeast growth than the antifungal agents you already tested in this experiment?
- You may have found in this experiment that some antifungal medicines are more effective at stopping yeast growth than others, when they are all used at the same concentration. But what is the lowest concentration at which each antifungal medicine needs to be used to completely stop yeast growth? Is it different for each antifungal medicine? You can investigate this using the same procedure.
- When you first did this experiment, if you found that the higher concentration completely stopped yeast growth for one of the antifungal medicines, but the lower concentration did not, try a concentration in between these two concentrations. If this in-between concentration still completely stops yeast growth, try a concentration in between this new one and the low concentration tested. Keep going until it does not completely stop yeast growth.
- This experiment uses yeast to test how effective different antifungal medicines are, but you can do many other experiments easily at home using yeast. Here are some additional Science Buddies projects and resources that use yeast:
Careers
If you like this project, you might enjoy exploring these related careers:
Contact Us
If you have purchased a kit for this project from Science Buddies, we are pleased to answer your questions.In your email, please follow these instructions:
- What is your Science Buddies kit order number?
- Please describe how you need help as thoroughly as possible:
Examples
Good Question I'm trying to do Experimental Procedure step #5, "Scrape the insulation from the wire. . ." How do I know when I've scraped enough?
Good Question I'm at Experimental Procedure step #7, "Move the magnet back and forth . . ." and the LED is not lighting up.
Bad Question I don't understand the instructions. Help!
Good Question I am purchasing my materials. Can I substitute a 1N34 diode for the 1N25 diode called for in the material list?
Bad Question Can I use a different part?
Contact Us
Related Links
- Science Fair Project Guide
- Other Ideas Like This
- Medical Biotechnology Project Ideas
- My Favorites
- Genetics Home Reference Tutorial
- NCBI Gene & SNP Tutorial
- PharmGKB Pharmacogenomics Knowledge Base Tutorial