Abstract
Have you ever tried to make parts of your hair lighter than the rest of your hair? Perhaps the way you tried to do it did not lighten it or maybe it turned out a weird orange color? With this science project you can understand why.Summary
Edited by Andrew Olson, Ph.D., Science Buddies
Sources
- Milady Publishing Company, 2000. Milady's Standard Textbook of Cosmetology Albany, NY: Milady Publishing Company, a division of Delmar Publishers, Inc.
- Nizetich, A., 1993. Milady's Teaching Hair Coloring: A Step-by-Step Guide to Building Props Albany, NY: Milady Publishing Company, a division of Delmar Publishers, Inc.
- Heavilin, S., ed., 2002. Milady's Illustrated Cosmetology Dictionary Albany, NY: Milady/Thomson Learning.
- The figures in the Introduction on human hair structure are from: Tobin, D.J., 2006. Biochemistry of Human Skin & Our Brain on the Outside. Chem. Soc. Rev., 2006, 35, 52-67. Retrieved July 15, 2021.

Objective
The goal of this project is to investigate how hydrogen-peroxide based hair treatments change the color of human hair.Introduction
Bleaching, lightening, or de-coloring (removing pigment) hair can be achieved by using natural sunlight or chemicals designed specifically for this purpose. Using sunlight alone, the results achieved will depend on the natural color of the hair. Visible results can take several weeks or months. If the natural hair color is darker than a medium blonde, the most successful way to lighten the hair is using hydrogen peroxide and an ammonia-based hair lightener.
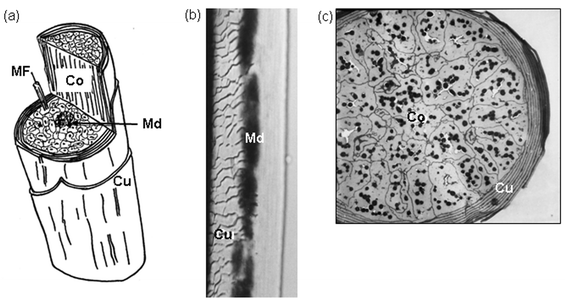
The inner structure of a strand of human hair is comprised of a small core called a medula that is surrounded by thick walls of cortical cells and microfibril. The entire hair shaft is also covered by a thin layer of cuticle cells.
Figure 1. Microscopic structure of a human hair shaft. Part (a) shows a cutaway cartoon of a single hair shaft. The labels show cuticle cells (Cu), cortical cells (Co), the medulla (Md), and a microfibril (MF) within a cortical cell. Part (b) shows a transmitted light micrograph of a single hair strand. The scale-like layer of cuticle cells (Cu) is clearly visible, as is the central medulla (Md). Part (c) shows a cross-section of a fine hair strand. The flattened cuticle cells (Cu) wrap tightly around the cortical cells (Co), which contain many dark pigment granules (Tobin, 2006).
The predominant proteins in hair are from the family of keratins, the same family of proteins that make your fingernails. Protein molecules are built from amino acids. In a hair strand, the keratin molecules contain a large number of a particular amino acid called cysteine. Each cysteine in the keratin molecule is a potential attachment point, where the keratin molecule can be tightly connected to another cysteine, forming a chemical bond called a cross-link. The keratins in hair have many such cross-links, making a hair strand strong and flexible. If you are interested in finding out about how hair grows, you should do research on hair follicles, the specialized structure in the skin that produces each individual hair strand.
The cuticle cells also have a coating of specialized molecules that repel water. These molecules are called lipids. By repelling water, the lipid molecules help to protect the hair strand. In order for bleaching chemicals to reach the pigment molecules in the cortical cells, the cuticle layer (including its protective lipid coating) must first be opened up. In chemical lightening solutions, this opening is accomplished by making the solution basic. You should do background research on the pH scale, to learn about basic, neutral, and acidic solutions. See the Bibliography for resources to get started.
The hair pigment goes through different stages of changing color as it lightens. The amount of change depends on how much pigment the hair has and the length of time the hair is exposed to the lightening chemicals. Lightening can be divided into roughly seven stages from the darkest to the lightest. A natural head of black hair will go from black to brown, to red, to red-gold, to gold, to yellow, and finally to pale yellow (almost white). The hair also becomes more porous (increasing the hair's capacity to absorb liquids) during the lightening treatment.
Hydrogen peroxide (H2O2) is an oxidizing chemical that bleaches the natural pigments in human hair. For hair treatment, the concentration of hydrogen peroxide is often expressed in volumes, referring to the total volume of oxygen (at standard temperature and pressure) that can be produced from the hydrogen peroxide. A "10 volume" solution is equivalent to 3% hydrogen peroxide in water (weight/volume, i.e., 3 grams of H2O2 plus enough water to make a total volume of 100 ml). A "20 volume" solution is equivalent to 6% hydrogen peroxide, etc. (Wikipedia contributors, 2006). The higher the concentration of peroxide used the greater the breakdown of melanin (tiny grains of pigment which create natural hair color) resulting in a lighter color.
Hair lighteners are available for use in liquid, cream, and powder form. By mixing a chosen concentration of hydrogen peroxide and a lightener, then applying the mixture to natural hair, we can achieve visible lightening of selected pieces of human hair. In this experiment, you will compare the results of lightening hair with a commercial product to untreated hair, and to hair treated with a "natural" hair lightener such as lemon juice or sunlight.
Terms and Concepts
To do this project, you should do research that enables you to understand the following terms and concepts:- Human hair strand, structure, composition, how it grows:
- Cuticle
- Cortex
- Pigments: eumelanin, phaeomelanin
- Keratin
- Hair follicle
- Understanding the pH scale is helpful for this project
- Hydrogen peroxide (H2O2)
Questions
- How are "volumes" of hydrogen peroxide related to concentration of hydrogen peroxide expressed in percentage terms (weight/volume)?
- How does the pH of the bleaching solution affect its ability to lighten hair?
- How does sunlight lighten hair color?
Bibliography
Most libraries should have copies of these books. (Note that each has multiple editions with different publication dates; the particular edition is not critical.)- Milady Publishing Company, 2000. Milady's Standard Textbook of Cosmetology Albany, NY: Milady Publishing Company, a division of Delmar Publishers, Inc.
- Heavilin, S., ed., 2002. Milady's Illustrated Cosmetology Dictionary Albany, NY: Milady/Thomson Learning.
- Science Buddies Staff (2012). Acids, Bases, and the pH Scale. Retrieved July 15, 2021.
- Helmenstine, A.M., Ph.D., 2007. Hair Color Chemistry, About: Chemistry, About, Inc., a part of The New York Times Company. Retrieved January 10, 2007.
- Wikipedia contributors, 2007. Peroxide, Wikipedia, The Free Encyclopedia. Retrieved January 10, 2007.
- Field, Simon Quellen, 2003. Ingredients: What's in the Stuff We Buy Kinetic MicroScience Press. Retrieved January 10, 2007.
- Tobin, D.J., 2006. Biochemistry of Human Skin & Our Brain on the Outside. Chem. Soc. Rev., 2006, 35, 52-67. Retrieved July 15, 2021.
Materials and Equipment
- Swatches of chemically untreated human hair (ideally at least 3 swatches from 3 different people, making 9 swatches total). The swatches should be:
- Approximately 1.5 cm wide x 10 cm long
- Light brown or darker in color
- "Chemically untreated," which means they have no permanent waves, no hair color, and no damage from styling with blow dryers and curling irons
- You should be able to obtain these from a beauty salon. You may need to go to a few beauty salons to find enough swatches.
- At least two different hydrogen peroxide-based developer volumes (at least 6 oz. each)
- Hydrogen peroxide-based developers come in "10 volume," "20 volume," "30 volume," and "40 volume" concentrations (which contains 3%, 6%, 9%, and 12% hydrogen peroxide, respectively). Pick at least two volumes to test in your experiment.
- These are the most commonly available volumes at beauty supply stores open to the public.
- You will need a parent or guardian to purchase.
- You could also ask at a beauty salon if the salon would be willing to help you with obtaining some.
- Hair lightener, powder or cream type (at least enough for one application on a typical head, which is the amount from one kit)
- Available at beauty supply stores open to the public.
- You will need a parent or guardian to purchase.
- You could also purchase a hair lightener (highlighting) kit from a superstore or drugstore.
- You could also ask a beauty salon if they would help you in obtaining these.
- Tint brush
- Available at a beauty supply store open to the public.
- Alternatively, you could use a stiff brush approximately 4 cm wide.
- Plastic or glass bowl. Make sure it has no metal.
- Aluminum foil
- Latex, vinyl, or polyethylene protective gloves. Can be purchased at a local drug store or pharmacy, or through an online supplier like Carolina Biological Supply Company. If you are allergic to latex, use vinyl or polyethylene gloves.
- Protective safety goggles or glasses. Chemical splash goggles can be purchased through an online supplier like Carolina Biological Supply Company.
- Optional: Lab coat. Can be purchased at scientific supply companies, or through an online supplier like Carolina Biological Supply Company. Alternatively, old, protective clothing may be used instead.
- Optional: If you want to compare "natural" lightening treatments, you may also need lemon juice.
- Lab notebook
Disclaimer: Science Buddies participates in affiliate programs with Home Science Tools, Amazon.com, Carolina Biological, and Jameco Electronics. Proceeds from the affiliate programs help support Science Buddies, a 501(c)(3) public charity, and keep our resources free for everyone. Our top priority is student learning. If you have any comments (positive or negative) related to purchases you've made for science projects from recommendations on our site, please let us know. Write to us at scibuddy@sciencebuddies.org.
Experimental Procedure
- Wear protective gloves and eyewear.
- The solutions can bleach your clothing if they splatter, so it's a good idea to wear a lab coat or old clothes.
- Avoid contact with skin and eyes. If contact occurs, immediately flush with lukewarm water.
- Obtain medical assistance for eye contact.
- Do your background research so that you are knowledgeable about the terms, concepts and questions, in the Background section. It is especially important that you research and understand the terms and structure of the human hair strand.
- Plan out how you will treat your swatches.
- You should ideally have 3 swatches from 3 different people, making 9 swatches total. You will need more swatches if you want to test more than two different developer volumes.
- For each group of 3 swatches from the same person, 2 swatches will be chemically lightened, and one will remain completely untreated for comparison. Each chemically lightened swatch should be treated using a different developer volume. For example, one may be treated with 10 volume, and the other using 20 volume.
- You should end up with 3 untreated swatches (each from a different person), 3 swatches treated with the same developer (each swatch from a different person), and 3 swatches all treated with a different developer (with each swatch from a different person).
- For chemically lightening a swatch of hair, use the following procedure:
- Secure one end of each hair swatch with an elastic band or sturdy tape.
- Wear protective gloves when mixing and using the hair lightener solution.
- Mix the hair lightener in a bowl.
- If you are using a kit, follow the directions on the lightening kit.
- If you are using powder lightener, use approximately 2 tablespoons of powder. Add enough hydrogen peroxide to make a creamy paste about the consistency of honey.
- If you are using a cream lightener, mix enough hydrogen peroxide to make a honey consistency.
- For a fair comparison use the same amount of hydrogen peroxide for each solution you make.
- Lay a hair swatch on a piece of aluminum foil.
- Apply the lightening mixture to the hair swatch with the stiff brush. Saturate the swatch with the mixture.
- Leave the mixture on the swatch for a set amount of time, for example, 30 minutes.
- Rinse the hair swatch with tap water.
- Dry the hair swatch.
- Remember to label each swatch and keep track of the treatment for each swatch (e.g., hydrogen peroxide concentration, lightener used, length of treatment time) in your lab notebook.
- For each group of swatches from the same person, be sure to keep one hair swatch completely untreated for comparison.
- As an optional comparison to peroxide treatment, you can try sunlight or another "natural" lightening treatment. You will need additional swatches to do this.
- For sunlight bleaching you can try this method:
- Put a swatch of hair in a secure outdoor location that receives direct sun (preferably afternoon).
- Do this for at least 1 week.
- Compare to untreated and chemically treated swatches and note your results.
- For sunlight bleaching you can try this method:
- For "natural" chemical lightening, you can try this method:
- Put enough lemon juice (or other natural lightener, such as chamomile tea) on the hair to saturate the hair swatch.
- Place in the sun (will not work without sunlight) for several hours.
- Rinse hair with tap water.
- Dry hair.
- Compare to untreated and chemically treated swatches and note your results.
- Analyze your results and try to draw conclusions about how the different developers change the color of human hair.
- Note that you can use the hair swatches on your science fair project display board to show the actual results of your experiment.
Ask an Expert
Variations
- Try different concentrations of hydrogen peroxide. What are the results in lightening the hair using "10 volumes," "20 volumes," and "40 volumes" peroxide?
- Are the results different if the lightener is left on the hair a longer time?
- What happens if the natural lightener or the mixture dries out on the hair? Does drying out stop the process of lightening?
- Compare effects on different types of hair. What is the degree of lightening on hair that is very dark (i.e., dark brown or black)? What is the degree of lightening on hair that is light to begin with (i.e., medium to light blonde)?
- Does adding heat (heat lamp) make a difference in the time or lightening of the hair?
- What condition is the hair left in after the lightening? Dry, rough, dull, or possibly destroyed? Does the length of time that the hair is exposed to the lightener solution affect the condition of the hair? Does a different concentration of peroxide affect the condition of the hair? Design an experiment to find out.
- Since chemical treatments must open the cuticle layer for the bleaching molecules to reach the pigment in the cortical layer, do chemical treatments permanently alter the structure of hair? One possible method for investigating this question is to build hygrometers using human hair. A hygrometer is a device used by weather forecasters to measure the relative humidity of the air. Human hair strands expand when the humidity is higher, and contract when the air is dryer. See the Science Buddies project Does Chemical Lightening Affect the Structure of Human Hair?. You will compare hygrometers made with untreated hair strands to hygrometers made with hair subjected to chemical lightening treatments. Can you see any differences between the treated and untreated hair?
Careers
If you like this project, you might enjoy exploring these related careers:
Related Links
- Science Fair Project Guide
- Other Ideas Like This
- Chemistry Project Ideas
- Cosmetic Chemistry Project Ideas
- My Favorites
- Chemistry Safety Guide
- Visualizing Molecules in Three Dimensions









