Abstract
Did you know that your body has a built-in cooler? And it might not be what you think! Sweat is produced when you are hot, but its purpose is actually to cool your body as the water in it evaporates from your skin. In this science fair project, you'll use the energy produced when water evaporates to cool down chocolate-covered candy so it doesn't melt.Summary
Kelsey Woods, Cyberchase Intern
Edited by Sandra Slutz, PhD, and Sabine De Brabandere, PhD, Science Buddies
The inspiration for this science fair project is the episode "Digit's B-Day Surprise" from CYBERCHASE on PBS KIDS GO!:
- Thirteen/WNET New York. (2008). CYBERCHASE: Episode 601: Digit's B-Day Surprise Educational Broadcasting Corporation, New York. Retrieved February 13, 2009.

Watch CYBERCHASE on PBS KIDS GO! Check local listings or visit pbskidsgo.org/cyberchase. CYBERCHASE is produced by THIRTEEN in association with WNET. All rights reserved. CYBERCHASE is a trademark of THIRTEEN. The PBS KIDS GO! logo is a registered mark of PBS and is used with permission.

Objective
In this science fair project, you will discover how to use the evaporation of water to keep chocolate-covered candy from melting.Introduction
You've probably noticed that when you're outside on a hot summer day, your body starts to sweat. But did you know that sweat, or perspiration, is actually your body's way of cooling down? Sweat, which is mostly water, cools us down when it evaporates.
Evaporation is the process that occurs when water changes from a liquid into a gas (in this case, the gas is water vapor). When your sweat evaporates, it carries heat energy from your body with it. The faster your sweat evaporates, the more heat is carried away, and the more the skin surface from which it evaporates is cooled.
As water evaporates from an object, it makes the air above the object more humid, (filled with more water vapor) which, in turn, slows down the evaporation process. This is because once the air is already full of water vapor, there is nowhere for the water on your skin to evaporate. But if you fan the moist, humid air away, then the water can evaporate more quickly. That's why you feel cooler if you fan yourself or if there's a gust of wind.
In places with hot weather, engineers design misters—machines that spray a fine water mist—for use in public places to help people keep cool. These misters help out your body's natural sweat cooling system by providing more water to evaporate and carry away heat energy from your body. Another way that you can keep cool on a hot day is by dipping a bandana in water and wearing it around your neck. The extra water from the bandana causes more evaporation, which keeps your body even cooler than it would be with only sweat.
You can apply the same process of evaporative cooling that your body uses in order to cool down objects. In the CYBERCHASE episode, "Digit's B-Day Surprise," the CyberSquad must use evaporative cooling to keep Digit's chocolate sculpture birthday present from melting while traveling through the desert. Click on the video box above to watch the CyberSquad use the power of evaporative cooling to save Digit's birthday present.
In the Cyberchase For Real segment, wilting in the heat as he tries to play tennis, Harry loses repeatedly to his obnoxious cousin Harley. After Harry realizes that Harley is keeping himself cool using water from a spray bottle with a fan attached, Harry decides to up his game. With a large sprinkler hose and three window fans, he harnesses the power of evaporative cooling on his side of the court, and defeats Harley without breaking a sweat.
How can you use evaporative cooling to keep chocolate candies from melting? This science fair project will help you find out!
Terms and Concepts
- Perspiration
- Evaporation
- Gas
- Water vapor
- Humidity
- Evaporative cooling
Questions
- How does sweat cool your body down?
- What are some ways people use evaporation to keep cool?
- How do engineers use evaporation to keep objects cool?
Bibliography
This science fair project is based on the following episode from CYBERCHASE on PBS KIDS GO!:
- PBS Kids Go! Cyberchase. (2008). Episode Descriptions: Episode 601: Digit's B-Day Surprise. Thirteen/WNET. Retrieved February 27, 2012.
- PBS Kids Go! Cyberchase. (2007). Taking the Temperature in Sensible Flats (Episode 601: Digit's B-Day Surprise). Thirteen/WNET. Retrieved February 27, 2012.
Check out this website to learn more about evaporation and the water cycle:
- USGS. (2008). The Water Cycle: Evaporation. Retrieved February 13, 2009.
Learn more about sweat from this website:
- Gavin, Mary L. (2006, May). What's sweat?. Retrieved February 13, 2009.
This website offers help with creating graphs:
- National Center for Education Statistics. (n.d.). Create a Graph. Retrieved February 22, 2009.
Materials and Equipment
- Paper towel (6 sheets)
- Scissors
- Small bowl of room-temperature water
- Chocolate candies in wrappers or small chocolate candy bars in wrappers (6) Note: Teardrop-shaped chocolates are not recommended.
- Tape
- Ruler
- Drinking glass
- Hair dryer
- Timer
- Lab notebook
Experimental Procedure
- Cut your paper towel sheet into strips that are about 3 inches wide.
- Take one paper towel strip and wet it by dripping water on it. It should feel wet, but not dripping.
- Keeping the candies in their wrappers, tightly wrap one of the candies in the wet paper towel strip and tightly wrap another candy in a dry paper towel strip. The dry paper towel strip might have a tendency to unwrap. Keep it in place with a small piece of tape.
- Place the two candies side-by-side on a heatproof surface. Place a glass upside down on the edges of the paper strips to keep the candy in place. The setup is shown in Figure 1.
 Image Credit: Sabine De Brabandere, Science Buddies / Science Buddies
Image Credit: Sabine De Brabandere, Science Buddies / Science Buddies
Figure 1. An upside-down glass holds the candies that are wrapped in paper towel strips in place.
- Hold your hair dryer so the air will blow down over the candies. The hair dryer should be 8 inches above the candy, as shown in Figure 2.
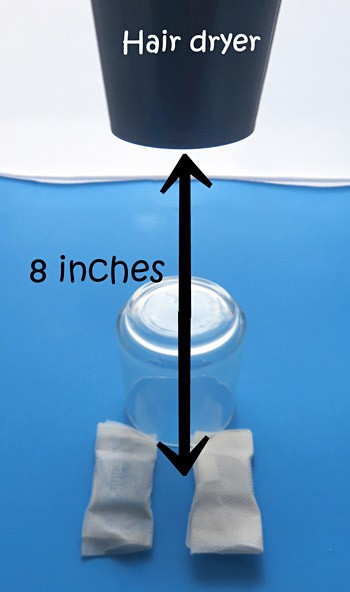 Image Credit: Sabine De Brabandere, Science Buddies / Science Buddies
Image Credit: Sabine De Brabandere, Science Buddies / Science Buddies
Figure 2. A hair dryer held at 8 inches above the candy blows hot air on the candies.
- Using your timer, blow hot air (with the hair dryer on high) for 5 minutes.
- After 5 minutes, stop the hair dryer. Observe how the paper towel strips have changed. Remove the paper towel strips and open the wrappers.
- In your lab notebook, record your observations about what happened to the candy wrapped in the wet paper towel versus the candy wrapped in the dry paper towel. A table like Table 1 can help organize your observations.
| Candy Wrapped in Dry Towel | Candy Wrapped in Wet Towel | |
|---|---|---|
| First trial | ||
| Second trial | ||
| Third trial |
- Repeat steps 1–8 two more times with new candies and paper towel strips. Are your observations consistent between trials? Can you use the information you learned from the Introduction and your background reading to explain your observations?
- To gain an even deeper understanding of evaporative cooling, try the Variations.
Ask an Expert
Global Connections
The United Nations Sustainable Development Goals (UNSDGs) are a blueprint to achieve a better and more sustainable future for all.
Variations
- Try the experiment again, using thermometers to determine the starting temperature, and the final temperature that each candy reaches after 5 minutes beneath the hair dryer. Graph your results. Note: for help creating graphs, try the Create a Graph website.
- What happens if you put the candies behind a window in the sun? The sun provides a heat source but the moist air would not be blown away like it is with a hair dryer. How does this affect the melting of the candies? What about the final temperature of the candies? Now what happens if you use a paper fan to fan the paper towel-wrapped candies while they are in the sun?
Careers
If you like this project, you might enjoy exploring these related careers:












