Abstract
The insides of a car engine get very hot when the engine is running. Motor oil lubricates the moving parts, to keep the engine operating smoothly. What happens to motor oil as the engine temperature goes up?Summary
Andrew Olson, Ph.D., Science Buddies
Sources
This project is based on:
- Ravanbakhsh, R., 2003. The Viscosity of Motor Oil, California State Science Fair Abstract. Retrieved November 2, 2006.
Objective
The objective of this project is to measure how the viscosity of motor oil changes with temperature.
Introduction
The internal combustion engine that powers your family's car has moving parts that work at high temperatures for billions of cycles over its lifetime. In order to keep the pistons moving smoothly in the cylinders, there is a thin film of motor oil between the piston rings and the cylinder wall. The oil is a lubricant—a slippery liquid that allows the piston to slide freely within the cylinder. Without oil, the piston would not be able to move in the cylinder—the engine would seize up and be ruined.
The primary functions of motor oil are:
- Lubricate engine moving parts to reduce friction and prevent wear.
- Clear engine of contaminants.
- Seal piston and liner for optimum engine efficiency.
- Resist high temperature degradation.
- Promote low temperature lubrication.
- Lubricate over a wide temperature range." (Thibault, 2001)
The oil must be able to keep the piston moving smoothly at cold temperatures (when the engine first starts up) and also at the normally high operating temperature of the cylinders.
One way of measuring an oil's ability to lubricate is to measure its viscosity. The viscosity of a fluid is a measure of the fluid's resistance to flow. You can think of it as fluid friction. Water is an example of a fluid with low viscosity—it pours easily and quickly. Cooking oil has a higher viscosity—it pours more slowly than water. For an engine lubricant, it is important that the viscosity does not change significantly as the temperature increases.
Viscosity of liquids can be measured with a special piece of glassware called a viscometer (see Figure 1). Fluid is drawn up from the cup on the lower left into the tube on the right, using a suction bulb. The suction is removed, and the time it takes for the fluid to drain out is measured. The higher the viscosity, the longer it will take the fluid to drain through the tube. To measure viscosity at different temperatures, the viscometer is placed in a water bath. Because viscometers are expensive (more than $170), you'll use a slightly different technique for this experiment.
 Image Credit
Image Credit
Figure 1. Ostwald-type viscometer for measuring viscosity of liquids.
Instead of using fancy glassware, you can use a Pyrex beaker or graduated cylinder. You will release a sphere (glass marble or steel ball bearing) at the surface of the liquid and time how long it takes for the sphere to fall to the bottom of the beaker or cylinder. You can make your comparisons using the measured time for the sphere to fall, or you can take your project a little further and actually calculate the viscosity. It's up to you.
In order to calculate the viscosity, you'll have to measure the time it takes the sphere to fall, the distance the sphere falls, and the density of the sphere and the fluid.
The equation (Equation 1) shows you how to calculate the viscosity from your measurements. It may look intimidating at first, because it has some Greek letters in it, but don't let that scare you. The variable commonly used to represent viscosity is the Greek letter "eta" (η). The variable commonly used to represent density is the Greek letter "rho" (ρ). The capital Greek letter "delta" (Δ) is often used as shorthand for taking the difference of something. The other variables in the equation are g, for the accleration due to gravity (980 cm/s2), a for the radius of the sphere (in cm), and v for the average velocity of the sphere as it falls through the fluid (in cm/s). The result is in units of poise (g/cm·s).
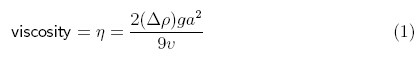
So the equation tells you to take the density of the sphere minus the density of the fluid (Δρ), multiply this by 2ga2, and then divide the result by 9v. If the sphere falls more quickly (i.e. with greater velocity), v is greater and η, the viscosity, is smaller, as we would expect. Conversely, if the sphere falls more slowly, the viscosity is greater. You would also expect a sphere that has higher density (i.e., is less buoyant) would fall faster than a sphere with lower density (i.e., is more buoyant). The density factor in the equation accounts for this. The amount of friction that the sphere experiences as it falls will be related to its surface area, which is proportional to the square of the sphere's radius.
You can use a water bath to heat or cool the oil to different temperatures in order to see how its viscosity changes with temperature. The Experimental Procedure section has more detailed instructions.
Terms and Concepts
- Viscosity
- Lubricant
- Internal combustion engine
Questions
- What do the SAE ratings on motor oil mean?
- Why is it important for motor oil to maintain its viscosity over a wide range of temperatures?
Bibliography
- What do the SAE ratings on motor oil mean? Find out from this article:
Thibault, R., 2001. How To Read an Oil Can, Machinery Lubrication Magazine, May 2001. Retrieved November 2, 2006. - This article describes a method for measuring viscosity of a liquid:
HSGC, 1996. Viscosity: Teacher Page, Hawaii Space Grant Consortium. Retrieved November 2, 2006. - For more basic information on viscosity, including the viscosities for several different liquids, see:
Wikipedia contributors, 2006. Viscosity, Wikipedia, The Free Encylcopedia. Retrieved November 2, 2006. - For a more advanced article on the physics of viscosity, see:
Transtronics, 2006. Viscosity, Transtronics. Retrieved November 2, 2006. - For information on how internal combustion engines work, see:
Brain, M., 2006. How Car Engines Work, Howstuffworks.com. Retrieved November 2, 2006.
Materials and Equipment
- Various motor oils with different viscosity ratings, e.g.:
- 5W-30
- 10W-40
- 20W-50
- Small sphere, e.g.:
- Glass marble or steel ball bearing
- Gram scale for measuring weight of oil and sphere, such as the digital pocket scale from Amazon.com
- Pyrex beaker or graduated cylinder (inside diameter must be larger than sphere),
- Tongs (for retrieving sphere from heated oil)
- Thermometer (0 to 100°C)
- Stop watch
- Large pan
- Hot plate or electric stove
- Water
- Ice
Disclaimer: Science Buddies participates in affiliate programs with Home Science Tools, Amazon.com, Carolina Biological, and Jameco Electronics. Proceeds from the affiliate programs help support Science Buddies, a 501(c)(3) public charity, and keep our resources free for everyone. Our top priority is student learning. If you have any comments (positive or negative) related to purchases you've made for science projects from recommendations on our site, please let us know. Write to us at scibuddy@sciencebuddies.org.
Experimental Procedure
- Safety Note: Use a hot plate or electric stove burner for heating the water bath in this experiment, never an open flame. Motor oil is combustible. Do not use it near an open flame. Also, at the conclusion of the experiment, be sure to dispose of the used motor oil properly.
-
Next you need to measure the time it takes for the sphere to fall through the oil.
- Pour the oil into the pyrex beaker (or graduated cylinder).
- Measure the height of the oil (in cm).
- Hold the sphere (marble or ball bearing) at the surface of the oil.
- Release the sphere and start the stopwatch at the same moment.
- Stop the stopwatch at the moment the sphere touches the bottom of the beaker (or graduated cylinder).
- Repeat the measurement several (at least 3) times. Remove the sphere with tongs, allowing the oil to drain off. Wipe the sphere dry with a paper towel. Check to make sure that the height of the oil has not changed.
- Cool the oil by placing the beaker (or graduated cylinder) in an ice bath. Stir the oil occasionally to insure uniform temperature. Check the temperature of the oil every ten minutes or so. When the temperature of the oil is no longer changing, record the temperature of the oil and repeat the velocity measurement as before.
- Heat the oil by placing the beaker into a pan of water on a hot plate or electric stove. Use medium heat. You do not want the water bath to boil vigorously. Stir the oil occasionally to insure uniform temperature. Check the temperature of the oil every ten minutes or so. When the temperature stops changing, record the temperature of the oil and repeat the velocity measurement as before.
- Calculate the average falling time for each temperature. To compare your results visually, you can make a bar graph of falling time (in seconds, on the y-axis) vs. temperature of the oil (in °C, on the x-axis). Or, you can go on to calculate the actual viscosity of the oil at each temperature by following the remaining steps.
-
Measure density of the sphere. Density is the weight per unit volume, in g/cm3. First measure the weight of the sphere in grams. To measure the volume of a sphere, measure how much water the sphere displaces in a graduated cylinder.
- Partially fill a graduated cylinder with water and note the water level (in mL, which is equivalent to cm3). Always measure the water level from the bottom of the meniscus (the curved surface of the water inside the cylinder).
- Tip the graduated cylinder sideways and gently roll the sphere into the cylinder.
- Write down the new water level, and subtract the original water level. The difference is the volume of the sphere (in cm3).
- To measure the density of the oil, first measure the weight of the empty graduated cylinder. Then pour oil into the cylinder and weigh the cylinder and oil together. Subtract the weight of the empty cylinder to get the weight of the oil. Read the volume of oil from the graduated cylinder. The density is the weight divided by the volume. Does the density of the oil change with temperature? Measure it and find out. Be very careful when pouring the heated oil! To be as accurate as possible, the graduated cylinder should be at the same temperature as the oil.
- Calculate the average velocity of the sphere at each temperature. The velocity is the distance that the sphere fell (in cm) divided by the average time it took to fall (in s).
-
Use Equation 1 to calculate the viscosity of the oil at each temperature.
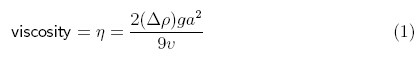
- Δρ = density of the sphere − density of the oil (in g/cm3),
- g = acceleration due to gravity (980 cm/s2),
- a = radius of the sphere (in cm),
- v = average velocity of the falling sphere (in cm/s).
- How does viscosity change with temperature?
Ask an Expert
Global Connections
The United Nations Sustainable Development Goals (UNSDGs) are a blueprint to achieve a better and more sustainable future for all.
Variations
- Do you get the same final viscosity if you use spheres of different densities (e.g., glass marble vs. steel ball bearing)?
- Does the viscosity of different motor oils share the same temperature dependence? In other words, if you make graphs of viscosity vs. temperature for different motor oils, do they all have the same slope?
- Measure viscosity of different cooking oils at different temperatures. Do cooking oils maintain their viscosity at elevated temperatures? How do they compare to motor oil in this regard? Why?
- Does the viscosity of motor oil change after it has been used? Measure the viscosity of used motor oil from your family's car, compared to brand new motor oil.
Careers
If you like this project, you might enjoy exploring these related careers: