Abstract
Are all reds the same? Find out in this science fair project! Investigate if the pigments in one type of red flower are different from those in another type of red flower. Flowers contain an assortment of amazing chemicals that produce color. In this plant biology project, you will analyze the colored pigments in different plants' red flower petals using paper chromatography, and compare the pigments in the different flowers.Summary
Teisha Rowland, PhD, Science Buddies
Edited by Svenja Lohner, PhD, Science Buddies
Objective
Analyze the pigments in red flower petals and determine if different red flowers use the same or different pigments.
Introduction
Plants contain many complex chemicals that have different functions. Some of these chemicals are used in the normal metabolism of the plant, such as those involved in photosynthesis. Some are involved in the plant's defense against insects, bacteria, and other pathogens. And some, such as the pigments in a flower, are involved in attracting the attention of possible pollinators, such as honeybees, butterflies, and hummingbirds.
There are two major classes of flower pigments: carotenoids and flavonoids. Carotenoids include carotene pigments, which produce yellow, orange, and red colors, and xanthophyll pigments, which only produce yellow colors. Flavonoids include anthocyanin pigments, which produce red, purple, magenta, and blue colors, and flavones and flavonol pigments, which produce yellow colors. Some flowers may even have chlorophyll, a green pigment usually found in the leaves of plants. Watch the video below to find out more about pigments in plants.
Usually, the color of the flower depends on the color of the pigments found in the flower. But there was a century-old mystery that defied this logic. Blue cornflowers appear to have the same pigments as red roses. How could this be explained? The pigment that is red in the rose is blue in the cornflower because the pigment in the cornflower interacts with other pigments and metals in the cornflower petal. This occurrence is a good example of how, in nature, new and unexpected characteristics can emerge from the organization of a few basic parts.
The goal of this plant biology science fair project is to analyze the pigments found in flowers using paper chromatography. To learn more about paper chromatography, you can continue reading and/or watch the video. The video gives an overview of what paper chromatography is, shows how it is done, explains the separation processes involved, and also provides tips and tricks for troubleshooting your experiment.
As mentioned in the video above, chromatography is a group of techniques, including paper chromatography, that are used to separate molecules in a complex mixture or solution, such as specific pigments in a mixture of pigments. In each chromatography apparatus there is generally a mobile phase, which is a fluid that runs along the stationary phase, and a stationary phase, that stays stationary while the mobile phase moves through. The mobile phase is also called the solvent.
In paper chromatography, a liquid like water or isopropyl alcohol is the mobile phase (or solvent), and chromatography paper, or more precisely the water embedded inside the paper, is the stationary phase. A mixture is dabbed onto a piece of chromatography paper near one end, at a point called the origin. The same edge as the mixture dab is immersed in the solvent, with the origin above the level of the solvent. The paper works like a wick, with the solvent moving up the paper, due to capillary action. The pigment molecules are then carried up the paper with the moving solvent.
How does the chromatography setup separate the components in the solution? The components ideally move at different speeds as they travel through the stationary phase. This is done by adjusting the mobile and stationary phases so that individual components of the mixture interact with both phases differently. Properties such as polarity, solubility, electrical charge, or other chemical properties usually determine how the components within a mixture are separated from each other. In paper chromatography, different pigments can be separated out from a solution based on the same principles. A pigment that interacts more with the mobile phase, for example because it is attracted more to the solvent than another pigment will generally travel farther because it will be easier for it to move in the mobile phase and be carried with the mobile phase along the stationary phase. A pigment that is less attracted to the solvent, or interacts more with the stationary phase than the mobile phase, will generally travel a shorter distance. Because different pigment molecules have different chemical properties, they are separated from each other on the chromatography paper, as shown in Figure 1 below.
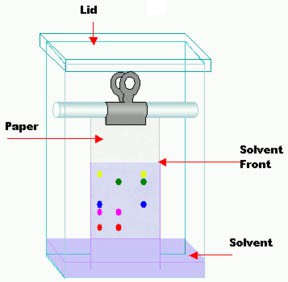 Image Credit: Wikipedia / Open source
Image Credit: Wikipedia / Open sourceA homemade paper chromatography testing box is made from a tall box with a lid. A dowel spanning the width of the box is placed near the top to allow a binder clip to hold a paper strip that has been marked by colored pigments. The paper strip is long enough to reach the bottom of the box where there is a small pool of solvent. As the solvent is absorbed by the paper and moves upward it brings some of the colored pigment markings with it.
Figure 1. Paper chromatography. Molecules are separated from each other, depending on how fast they migrate with the solvent up the chromatography paper. (Wikipedia, 2008.)
In paper chromatography, you can see the components separate out on the chromatography paper and identify the components based on how far they travel. To do this, we calculate the retention factor (Rf value) of each component. The Rf value is the ratio between how far a component travels and the distance the solvent (mobile phase) travels from a common starting point (the origin). For example, if one of the sample components moves 2.5 centimeters (cm) up the paper and the solvent moves 5.0 cm, as shown in Figure 2 below, then the Rf value is 0.5. You can use Rf values to identify different components as long as the solvent, temperature, pH, and type of paper remain the same. In Figure 2, the light blue shading represents the solvent and the dark blue spot is the colored solution sample.
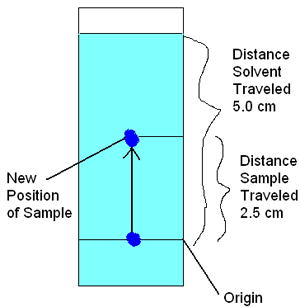
Diagram of a paper chromatography strip shows how marker ink travels up the length of a paper strip when the strip absorbs a liquid solvent. An origin line marks the original position of the ink and the solvent is colored blue so the distance the solvent travels up the paper strip can be measured. In the diagram, solvent traveled 5 centimeters, while the sample traveled 2.5 centimeters from the origin line.
Figure 2. For each compound, an Rf value is calculated based on how far it traveled along the stationary phase. In paper chromatography, Rf values are used to compare different components to each other.
Rf values are calculated by looking at the distance each component travels on the chromatography paper compared to the distance traveled by the solvent front. This ratio will be different for each component due to its unique chemical properties.
When measuring the distance the component traveled, you should measure from the origin (where the middle of the spot originally was) and then to the center of the spot in its new location. To calculate the Rf value, we then use Equation 1 below.
Equation 1:
In our example, this would be:
Note that an Rf value has no units because the units of distance cancel.
You can use Rf values to identify different pigment molecules as long as the solvent, pH, and type of paper remain the same.In this plant biology science project, you will use paper chromatography to analyze the pigments in red flowers from different species. Do you think they will have the same pigments, as determined by paper chromatography, or will the pigments be different?
Terms and Concepts
- Pigment
- Carotenoids
- Flavonoids
- Carotene
- Anthocyanin
- Paper chromatography
- Chromatography
- Solution
- Mobile phase
- Stationary phase
- Solvent
- Origin
- Capillary action
- Polarity
- Solubility
- Rf value
Questions
- Which pigments may cause red colors in flowers?
- In paper chromatography, how does the mobile phase travel through the stationary phase?
- How can paper chromatography separate different pigment molecules? How is solubility involved?
- What is a retention factor (Rf) and how can it be used to identify a specific pigment?
Bibliography
- Clark, Jim. (2007). Paper Chromatography. Retrieved March 18, 2013.
- WebExhibits. (n.d.). What pigments are in fruit and flowers?. Retrieved April 24, 2013.
- NBC News Learn. (2020, May 2). The Chemistry of Flowers. YouTube. Retrieved July 14, 2021.
These resources will give you more information about chromatography and teach you about the types of chromatography used in research labs today:
- Science Buddies. (n.d.). Paper Chromatography Resources. Retrieved January 14, 2018.
- Waters Corporation Staff. (2012). High Performance Liquid Chromatography. Retrieved November 16, 2023.
Materials and Equipment 
Recommended Project Supplies
- Candy Chromatography Science Kit, available from our partner
Home Science Tools.
You will need these items from the kit:
- Chromatography paper strips; 9. The kit comes with 20 strips; additional chromatography paper can be purchased separately from our partner Home Science Tools.
- 100 mL beaker
- 90% isopropyl alcohol
- Mini binder clips (2)
- Wooden splints
- Note: This kit contains additional items to do other chromatography science projects. See the kit instructions page for details. Downloadable materials safety data sheets (MSDS) are available for all kit chemicals if required by your science fair.
- You will also need to gather these items, not included in the kit:
- 100 mL graduated cylinder or measuring cup. A 100 mL graduated cylinder is available online at Amazon.com.
- Clean jar, drinking glass, or mug
- Scissors
- Ruler, metric
- Pencils (2)
- Distilled water (at least 60 mL). Distilled water is preferable, but tap water is also suitable
- Flower petals, red; you will need at least 2 flower petals from at least 3 different plants, which you can get from your own garden or from a florist or plant nursery. Tip: Larger petals, such as those from roses and tulips, work better than smaller petals.
- Piece of scratch paper
- Coin
- Timer or clock
- Lab notebook
Disclaimer: Science Buddies participates in affiliate programs with Home Science Tools, Amazon.com, Carolina Biological, and Jameco Electronics. Proceeds from the affiliate programs help support Science Buddies, a 501(c)(3) public charity, and keep our resources free for everyone. Our top priority is student learning. If you have any comments (positive or negative) related to purchases you've made for science projects from recommendations on our site, please let us know. Write to us at scibuddy@sciencebuddies.org.
Experimental Procedure
To make sure you can compare your results, as many of your materials as possible should remain constant. This means that the temperature, type of water used, size of paper strips, where the ink is placed onto the paper, etc., should remain the same throughout the experiment.
Cutting and Marking the Paper Strips
- Cut each chromatography paper in half (length-wise) to make approximately 2 centimeters (cm) wide by 7.5 cm long strips. You will need at least 9 chromatography strips.
- In your lab notebook, assign a number to each different type of flower, starting with the number 1 and going up. Using a pencil, number three strips "1," three other strips "2," and three other strips "3" at the top of the strip. This is so that you can identify which flower was used with which strip later.
- If you are investigating more than three different types of flowers, similarly continue to number the test strips.
- Draw a pencil line 1 cm from the edge of each strip of paper, as shown in Figure 3 below.
- This will be the origin line or baseline.
- You will spot the flower petal pigment for each strip right on this line, as shown in Figure 3.
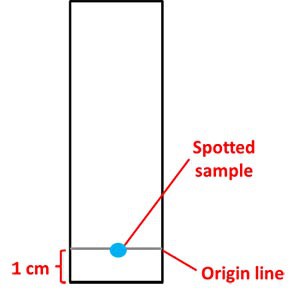 Image Credit: Sandra Slutz, Science Buddies / Science Buddies
Image Credit: Sandra Slutz, Science Buddies / Science Buddies
Figure 3. Each chromatography strip will have an origin line (baseline). The flower pigment to be tested will be spotted in the middle of the origin line.
Performing Paper Chromatography on the Paper Strips
- In your lab notebook, make a data table that lists the kinds of flowers you will be testing. Also make four columns titled something similar to "Color of the Pigment Band," "Distance to the Solvent Front (in cm)," "Distance to the Top of the Band (in cm)," and "Retention Factor (Rf)."
- In the jar, dilute the 90% isopropyl alcohol by mixing 75 milliliters (mL) of water with 30 mL of isopropyl alcohol. This water and isopropyl alcohol mixture is your solvent.
- Pour a small amount of the solvent into your 100 mL beaker, about 1–2cm cm deep.
- Next, transfer the pigments from one type of flower onto a strip of chromatography paper.
- Pick a flower type you want to investigate.
- Take one of the paper strips you prepared with this flower's number and place it on top of a piece of scratch paper on a hard surface. Note: Some pigments can stain so the paper strip should be prepared on a piece of scratch paper to protect the surface beneath it from getting stained.
- Lay a petal from the selected flower on the paper strip, over the origin line.
- Roll a coin, like a wheel, over the petal and across the origin line. Push down hard so that the petal is crushed and a strip of pigment is visibly transferred to the strip.
- Repeat step d about three to four times (using a fresh, unused part of the petal each time) so that a thick line of pigment is transferred to the strip, on the origin line. Be careful to only transfer the pigment onto the origin line.
- If the origin line becomes a little wider with pigment, this is OK, but make a note of it in your lab notebook.
- If you accidentally transfer pigment to an area that is away from the origin, prepare a new strip of paper as you did in the "Cutting and Making the Paper Strips" section and repeat steps 4b-e in the "Performing Paper Chromatography on the Paper Strips" section.
- Note Only prepare one strip with sample at a time so that the sample does not dry out. Otherwise, the pigment separation will not work.
- Clip the prepared chromatography strip to a wooden splint. Rest the splint on top of the beaker so that the strip hangs straight into the beaker. Note: The origin should not be immersed in the solvent.
- If necessary, add more of the solvent. The goal is to have the end of the chromatography strip just touching the surface of the solvent solution, as shown in Figure 4 below.
 Image Credit: Sandra Slutz, Science Buddies / Science Buddies
Image Credit: Sandra Slutz, Science Buddies / Science Buddies
Figure 4. Your setup should look similar to this example. The end of the chromatography strip should just touch the alcohol. Note: This picture does not show chromatography strips with flower pigments. The colors on your paper strips should look different.
- Let the solvent rise up the strip (by capillary action) until the solvent front is about 2 cm from the top and then remove the strip from the solvent. Check on the strip and the solvent front every 5 to 10 minutes—if you let it run too long the pigments may run off the strip and become distorted.
- Depending on the chromatography paper, this may take anywhere from 30 minutes to several hours.
- Be patient and do not take the paper strip out early. The longer you leave the paper strip in the solvent, the better your separation will be!
- Use a pencil to mark the solvent front.
- Allow the chromatography strip to dry. Tip: An easy way to do this is to tape the strip to the overhang of a counter or table so that the strip is dangling in the air.
-
After the strip has dried, measure the distance (in centimeters) from the origin to the solvent front and from the origin to the top of the pigment band that should be visible, as shown in Figure 5 below. Record the data in the data table you made in your lab notebook.
- Also record the color of the pigment band. For example, it may look "red," "purplish red," "pink," etc.
- Note: If you see more than one pigment band on the strip, record this band's color in your data table in a new column titled "Color of the Second Pigment Band." Also measure the distance from the origin to the top of this pigment band and record the data in your data table in a new column, titled "Distance to the Top of the Second Band (in cm)."
- Repeat steps 4–10 two more times for the same type of flower.
- Repeat steps 4–10 two more times, each time with a different type of flower, so that you have run at least three chromatography paper strips for each of the three different flower types you want to investigate.
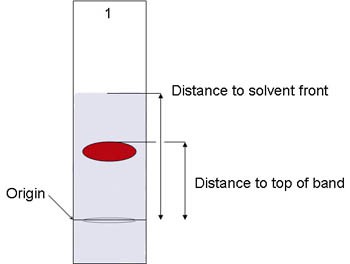 Image Credit: David B. Whyte, Science Buddies / Science Buddies
Image Credit: David B. Whyte, Science Buddies / Science BuddiesThe distance from the top of the red pigment to the origin line is divided by the distance of the solvent front to the origin line to solve for an Rf value.
Figure 5. The pigment moves up the paper as the solvent front advances. The Rf value is the ratio of the distance to the top of the band, to the distance to the solvent front, measured from the origin.
Analyzing Your Data
-
Now, using Equation 1 from the Introduction, calculate the Rf value for each pigment for each strip. Record the values in the data table in your lab notebook.
- Note: If you saw more than one pigment band on a strip, calculate the Rf value for this band as well and record it in your data table in a new column titled "Retention Factor (Rf) of the Second Band."
-
Compare the Rf values and colors of the pigment bands for each different flower. To be the same pigment, the pigment bands should have similar Rf values and be a similar color. Do all of the different red flowers you investigated have the same pigments, as determined by paper chromatography? Or did the different flowers have different pigments? If they used different pigments, was there one pigment that was in most of the red flowers?
- If one pigment was used by multiple red flowers, which pigment do you think it might be? Tip: You may want to re-read the Introduction in the Background section, and do additional research on carotene and anthocyanin pigments.
Ask an Expert
Global Connections
The United Nations Sustainable Development Goals (UNSDGs) are a blueprint to achieve a better and more sustainable future for all.
Variations
- Try this science project with other, similar colors of flowers, such as purple, pink, or orange flowers. Do they have pigments similar to the ones in the red flowers? You could even try it with a greater variety of flower colors, such as yellow, blue, or green flowers. What pigments do you think are in these flowers?
- Some plants grow very colorful leaves, such as coleus plants, bromeliads, and purple clovers. You could try this science project again but this time investigate colorful leaves on plants instead of flowers. What pigments make the leaves so colorful? Are these the same as the pigments in similarly colored flowers?
- Changing the mobile phase, or solvent, in paper chromatography can affect how well the chromatography works. Investigate this by trying other solvents, such as plain water, (undiluted) 90% percent isopropyl alcohol, isopropyl alcohol that has been diluted 90% with water, saltwater, nail polish remover, etc. Does a pigment travel different distances depending on the mobile phase you use? What do you think this tells you about the solubility of that pigment in the different mobile phases?
- For other Science Buddies science fair projects about paper chromatography, try
Careers
If you like this project, you might enjoy exploring these related careers:
Contact Us
If you have purchased a kit for this project from Science Buddies, we are pleased to answer your questions.In your email, please follow these instructions:
- What is your Science Buddies kit order number?
- Please describe how you need help as thoroughly as possible:
Examples
Good Question I'm trying to do Experimental Procedure step #5, "Scrape the insulation from the wire. . ." How do I know when I've scraped enough?
Good Question I'm at Experimental Procedure step #7, "Move the magnet back and forth . . ." and the LED is not lighting up.
Bad Question I don't understand the instructions. Help!
Good Question I am purchasing my materials. Can I substitute a 1N34 diode for the 1N25 diode called for in the material list?
Bad Question Can I use a different part?
Contact Us
Related Links
- Science Fair Project Guide
- Other Ideas Like This
- Plant Biology Project Ideas
- My Favorites
- Paper Chromatography