Others Like “Chemical Reactions That Change Back” (top 20 results)
|
Maple syrup on pancakes, ripe bananas, and soft drinks are all foods that are tasty to us
because of the sugar in them. But did you know there are different kinds of
sugar? One food can have multiple kinds of sugar in it, and our bodies actually process
the different types of sugars differently. In this science project, you will measure the
concentration of two sugars—glucose and sucrose—in different foods, and investigate how
sucrose is converted into glucose with the help…
Read more
Pennies are bright and shiny when they are new, but become quite dull with time. What causes such a drastic change? Oxygen in the air combines with the copper in the penny to form copper oxide, which makes the penny look dull and dingy. You can make the pennies look like new again by soaking them in water that is corrosive enough to strip off the copper oxide layer. It turns out, however, that the same process that makes the pennies shiny has bad consequences when it comes to copper pipes: it…
Read more
You might know that your body needs oxygen to keep going, and that you breathe out carbon dioxide as waste. What happens when you exercise? You have probably noticed that you breathe faster, and your heart beats faster. What triggers your body to respond in this way? How does it "rev up" to keep your muscles going? In this project, you will get a peek into the fascinating science of exercise physiology and find out—with the help of a color changing reaction.
Read more
Most of the ultraviolet (UV) light produced by the Sun is blocked by the atmosphere, but some UV light does still reach Earth. It can be detected using electronic devices, but can also be detected with something called UV beads. UV beads contain a pigment that changes color when they are exposed to ultraviolet radiation from the Sun. In this chemistry science fair project, you will use UV beads to study how temperature affects the rate at which they lose their color.
Read more
Which type of orange juice has the most vitamin C? In this science project, you will learn how to measure the amount of vitamin C in a solution using an iodine titration method. You will compare the amount of vitamin C in three different types of orange juice: homemade, premium not-from-concentrate, and orange juice made from frozen concentrate. Which do you think will have the most vitamin C?
Read more
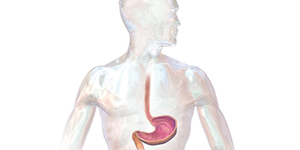
Have you ever experienced heartburn after eating a large, greasy meal? Or have you heard of someone complaining about heartburn pains? It's very common; over 60 million people in the U.S. have heartburn at least once every month. Heartburn pain usually results from stomach acids escaping from the stomach and irritating the esophagus above it. Luckily, there are medical drugs, called antacids, which can help relieve heartburn pain. But how do antacids work, and how effective are they? In this…
Read more
You may have seen police investigators on TV spraying a crime scene with a liquid that glows blue if there is any blood present. Luminol is the chemical which causes the glowing. In this chemistry science fair project, you will investigate what factors make this interesting molecule "light up."
Read more
Instant cold packs are popular with coaches and parents for treating minor bumps and bruises. The instant cold packs are not pre-cooled—you just squeeze the cold pack and its starts to get cold. So how does it work? In this chemistry science fair project, you will investigate the chemical reaction that occurs in instant cold packs.
Read more
Have you ever had a cut or a bloody nose that seemed like it would bleed forever? Though it might have seemed like a long time, it probably did stop pretty quickly. This is because different factors in a person's blood normally work together to plug the opening caused by the cut in a process called blood clotting or coagulation. However, some people have a genetic disorder called hemophilia that causes them to bleed excessively. If a person has hemophilia, he or she is usually missing some of…
Read more
Do you enjoy drinking smoothies packed full of berries and other tasty fruits? Or maybe you like drinking a creamy milkshake with peanut butter, chocolate, and bananas. Smoothies and milkshakes are often tasty to us because of the sugar in them. But did you know there are different kinds of sugar? Some ingredients in a smoothie can have more than one kind of sugar in them, and our bodies process each kind of sugar differently. In this science project, you will measure the concentration of…
Read more
|
Explore Our Science Videos
Make a Model to Explore the Distance from the Planets to the Sun
Popsicle Stick Paddle Boat
Squishy Circuits Classroom Activity Part 1