Water-Based Electricity?
I don't know what was being talked about, but as I cleaned up in the kitchen, I heard a conversation with an 8-year old that resulted in questions about water and electricity and whether or not water can conduct electricity. I think the question had come up as he relayed some cool feature of a character in a game and was challenged about whether or not such a power was possible.
Of course, as adults, we think of things like fallen power lines and rain puddles or the risk of a hair dryer falling in a bathtub. Those are the kinds of warnings I grew up with. Clearly, water can conduct electricity!
It's more complicated than that, of course. Water conducts electricity only if it's contaminated. "Pure" water, in other words, does not. But, for all extents and purposes, the feature in the game was likely feasible, and I still steer clear of hair driers and bathtubs.
This morning, I plugged the search terms into the Science Buddies site to see what projects might come up that would let me demonstrate this concept to a 2nd grader. I didn't find the perfect demonstration for the grade level, but a number of interesting projects for older students exist in the Science Buddies science fair project repository:
- Electrolyte Challenge: Orange Juice vs. Sports Drink
[http://www.sciencebuddies.org/science-fair-projects/project_ideas/Chem_p053.shtml]
Science Buddies' Difficulty Level 7-8 - Solar-Powered Water Desalination
[http://www.sciencebuddies.org/science-fair-projects/project_ideas/EnvEng_p022.shtml]
Science Buddies' Difficulty Level 6-7 - Conductance as a Water Quality Measurement
[http://www.sciencebuddies.org/science-fair-projects/project_ideas/Elec_p011.shtml]
Science Buddies' Difficulty Level 5
Do you have a favorite project for demonstrating these principles and introducing students of various ages to conductivity?
Categories:
You Might Also Enjoy These Related Posts:
- Plastics and Earth Day - Science Projects
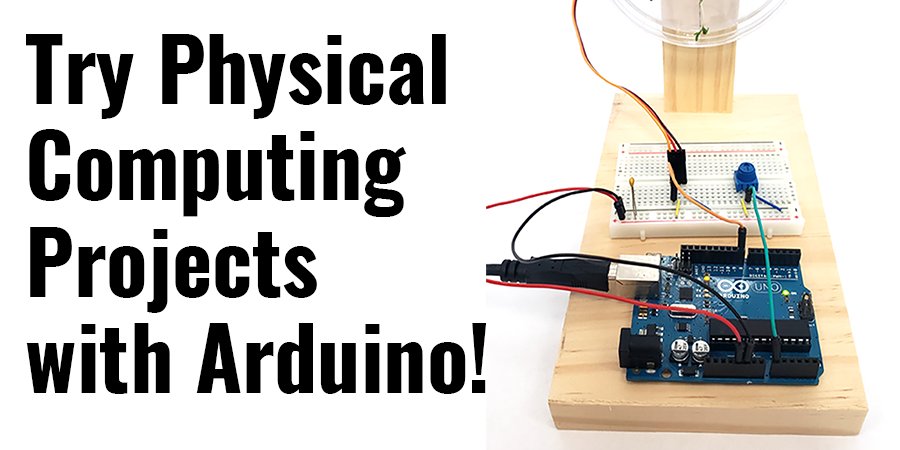
- Arduino Science Projects and Physical Computing
- 10+ Robotics Projects with the BlueBot Kit
- 5 STEM Activities with Marshmallow Peeps
- March Madness Basketball Science Projects: Sports Science Experiments
- Women in STEM! More than 60 Scientists and Engineers for Women's History Month
- Explore Artificial Intelligence and Machine Learning with Student AI Projects
- 10 Reasons to Do the Rubber Band Car Engineering Challenge