I Love Ice Cream, But It Doesn't Love Me: Understanding Lactose Intolerance
Abstract
What do pizza, milk shakes, and ice cream sundaes have in common, besides being delicious and loaded with calories? You might be surprised to learn that these foods, because they contain dairy products, cannot be eaten by the majority of people around the world. Dairy products contain the sugar molecule lactose, and the majority of people on the planet slowly begin to lose the ability to digest lactose after the age of 2. In this human biology and health science fair project, you will investigate the activity of lactase, the enzyme responsible for the ability to digest lactose.Summary
David Whyte, PhD, Science Buddies
This project is based on the following project: Kalumuck, K. (n.d.). Milk Makes Me Sick. Exploratorium Snacks. Retrieved July 10, 2008.
Recommended Project Supplies
Objective
The objective is to explore the biochemical basis for lactose intolerance. You will add the enzyme lactase to solutions containing the milk sugar lactose, and then test for one of the reaction products, glucose, using glucose test strips.
Introduction
The inability to digest lactose leads to lactose intolerance, which is a very unpleasant reaction to the presence of lactose in the digestive system, characterized by cramps, bloating, gas, and diarrhea.
Lactose is a key constituent of breast milk, so it is essential that babies are able to digest it, and they do. It accounts for approximately 40 percent of the total calories provided by breast milk. Babies are able to digest lactose because they produce lactase. Lactase is an enzyme that is present in the baby's digestive tract. Enzymes are protein molecules that function as catalysts, which vastly speed up the rates of chemical reactions.
Lactase catalyzes the breakdown of lactose into glucose and galactose as shown in Figure 1. Unlike lactose, glucose and galactose are readily absorbed by the small intestine.
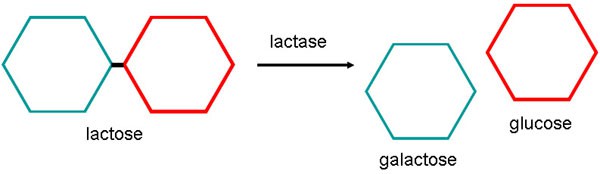 Image Credit: Wikipedia / Open
Image Credit: Wikipedia / Open
Figure 1. The enzyme lactase catalyzes the breakdown of lactose into the smaller sugars, galactose and glucose.
For reasons that are not all that clear, people stop producing lactase after about age 2. Once the production of lactase stops, drinking milk or eating milk products, such as cheese, can cause uncomfortable digestive problems. In people with low levels of the enzyme lactase, the lactose sugars in milk pass through the gastrointestinal tract undigested or are partially digested by enzymes produced by intestinal bacterial flora to yield short-chain fatty acids, hydrogen, carbon dioxide, and methane. These undigested lactose molecules and products of bacterial digestion result in the symptoms of lactose intolerance.
For those who have trouble digesting food containing dairy products, the lactase enzyme is available in tablet form. The tablets are taken with the first bite of dairy food. The lactase enzyme in the tablet breaks down lactose in the dairy product, making the food easy to digest. The lactase enzyme is also available as a liquid. Adding a few drops of the enzyme to milk reduces the level of lactose, making the milk more digestible for people with lactose intolerance. Lactose-reduced milk is available at most grocery stores. The milk contains all of the nutrients found in regular milk, but the level of lactose has been reduced.
Lactose intolerance is not a rare syndrome; it is, in fact, "normal" in the sense that the majority of people around the world are lactose intolerant. Lactose tolerance—the ability to digest dairy products—is present in a minority of the world's population, and is associated primarily with people whose ancestry is derived from western or northern Europe.
Lactose tolerance is due to a genetic change that occurred a few thousand years ago in northern Europe. This genetic change resulted in the maintenance of lactase production into adulthood. The current scientific consensus is that this mutation was advantageous and thus able to spread rapidly through the population of Europe.
Between 30 and 50 million Americans are lactose intolerant (total population: ~ 305 million) and certain ethnic and racial populations are more affected than others. Up to 80 percent of African Americans, 80-100 percent of American Indians, and 90-100 percent of Asian Americans are lactose intolerant. The condition is least common among people of northern European descent.
Lactose intolerance is a fascinating subject because it involves a number of areas of scientific inquiry, including genetics, anthropology, and enzymology. In this human biology science fair project, you will use lactase to catalyze the breakdown of lactose in milk into glucose and galactose. The level of glucose that is formed by the activity of the lactase enzyme depends on the initial level of the lactose sugar in the milk. In other words, to determine the level of lactose in the milk, you will first convert it to glucose and galactose, then measure the level of glucose. You will use glucose test strips to test the level of glucose formed by the breakdown of lactose. In the variations, you can explore the enzymology of lactase activity in more detail.
Terms and Concepts
- Lactose
- Lactose intolerance
- Lactase
- Enzyme
- Catalyst
- Glucose
- Galactose
- Lactose tolerance
- Enzymology
- Positive control
- Negative control
Questions
- What fraction of the world's population is lactose intolerant?
- What is the genetic basis for lactose tolerance?
- What are some theories as to why humans become lactose intolerant after infancy?
- The glucose test strip changes color in the presence of glucose. What happens on the strip to cause this change in color?
- How is regular milk treated to make it lactose-free?
Bibliography
- Patterson, K.D. (2008). Lactose Intolerance. The Cambridge World History of Food. Retrieved July 14, 2008.
- Sibley, E. (2008). Lactose Intolerance. National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. Retrieved July 15, 2008.
Materials and Equipment 
Recommended Project Supplies
- Sugar Metabolism Kit, available from our partner
Home Science Tools.
You will need these items from the kit:
- Graduated cylinder, 100 mL (1)
- Glucose powder (30 g) (1)
- Urinalysis test strips that measure glucose
- You will also need to gather these items, not included in the kit:
- Small plastic or glass cups
- Measuring cup (metric) or beaker
- Spoon
- Permanent marker
- Regular milk (the percent fat should not matter, but you should use the same percent fat for the regular and lactose-free milk)
- Lactose-free milk (the percent fat should not matter, but you should use the same percent fat for the regular and lactose-free milk), should be 100% lactose free; available at most grocery stores
- Lactase drops; if you can not find these at your local drug store or grocery store, you can purchase them online from Amazon.com
- Scale, must be able to accurately measure 2 g. A digital scale accurate to at least 1 g (the Fast Weigh MS-500-BLK Digital Pocket Scale) is available from Amazon.com.
- Stopwatch
- Room-temperature tap water
- Lab notebook
Disclaimer: Science Buddies participates in affiliate programs with Home Science Tools, Amazon.com, Carolina Biological, and Jameco Electronics. Proceeds from the affiliate programs help support Science Buddies, a 501(c)(3) public charity, and keep our resources free for everyone. Our top priority is student learning. If you have any comments (positive or negative) related to purchases you've made for science projects from recommendations on our site, please let us know. Write to us at scibuddy@sciencebuddies.org.
Experimental Procedure
The first step is to make positive and negative control solutions. The positive control solution is 2% glucose in water. You will use the positive control to make sure the glucose test strips are able to detect glucose. The negative control is just water. You will use the negative control to make sure the glucose test strips do not react to plain water.
- Make the positive control solution of 2% glucose in water.
- Label a small plastic cup with Positive Control.
- Use a scale to weigh 2 g of glucose powder into the plastic cup.
- Add 100 mL of water and stir until the glucose has dissolved.
- Fill another small cup with plain water and label it Negative Control.
- Test the positive and negative control solutions with the glucose test strips.
- Dip separate test strips into the glucose solution (positive control), and water (negative control).
- After 1–2 seconds, remove the test strips from the solution and wait for the length of time specified by the strip directions (30 seconds).
- Record any color changes of the strip and compare them to the key on the bottle, shown in Figure 2, to determine the glucose concentration of the tested fluid. Record your observations in your lab notebook.
- You may want to take a picture of the test strips and use them as part of your science project display board.
 Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Figure 2. This is the color chart for glucose on the test strip bottle. After a glucose test strip has been dipped in a glucose solution and removed, its color should change and match a color on its bottle (or be between two colors). The color on the bottle will indicate the percentage of glucose in the solution tested.
- You should see a clear positive reaction for the 2% glucose control and a clear negative reaction for the water control. Note: If you do not have a clear color change for the positive control solution try steps 1–3 again. If it is still problematic the second time, you might have to buy new test strips. It is okay to have a slightly lower reading for the pure glucose solution. Remember, these test strips were designed for measuring low concentrations of glucose in a urine sample, so the results might be slightly different for pure glucose solutions.
The next step is to determine the level of glucose in both, regular milk and lactose-free milk. Can you predict which one will have a higher glucose level?
- Prepare your lactose-free and regular milk samples.
- Label two small cups with lactose-free milk and regular milk.
- Use the measuring cylinder to measure out 20 mL of each milk and and pour each milk (lactose-free and regular) in the corresponding cup. Note: Rinse the measuring cylinder with water in between.
- Determine the glucose concentration in the regular milk sample.
- Following the directions that came with the glucose test strips, dip a strip into the milk sample. After 1–2 seconds, remove the test strip from the milk sample and wait for the length of time specified by the strip directions (30 seconds).
- Once the wait time has passed, compare the color of the test strip with the color-coded key on the side of the bottle, shown in Figure 2, to determine the concentration of glucose in the milk.
- Note: You may want to take a picture of the test strips and use them as part of your science project display board.
- Determine the glucose concentration in the lactose-free milk, using a fresh test strip.
- Record your results in your lab notebook.
What will happen when you add the enzyme lactase to the regular milk and to the lactose-free milk?
- Add one drop of the lactase solution to the regular milk sample.
- Warm the milk by rolling the cup back and forth in your hands for 2 minutes. Use the stopwatch to time yourself.
- Repeat the glucose test with a fresh test strip.
- Record your result in your lab notebook.
- Add one drop of the lactase solution to the lactose-free milk sample.
- Again, warm the milk by rolling the cup back and forth in your hands for 2 minutes. Repeat the glucose test with a fresh test strip.
- Record your results in your lab notebook.
- Repeat steps 5–15 two more times so that you have three trials for each milk sample, both before and after adding lactase. Use fresh cups or be sure to clean out your cups carefully before you begin new trials. Always use fresh glucose test strips.
- Explain your results.
- Is there a difference in glucose concentration between regular milk and lactose-free milk before addition of the lactase? Why?
- Do the glucose concentrations of regular milk and lactose-free milk change after the addition of the lactase? Why?
Ask an Expert
Global Connections
The United Nations Sustainable Development Goals (UNSDGs) are a blueprint to achieve a better and more sustainable future for all.
Variations
- Besides cow's milk, what other foods (goat's milk, soy milk, etc.) have lactose? Design tests to determine if the products actually contain lactose.
- Try to get more accurate, quantitative readings for the glucose level in your test solutions. You can make a series of glucose solutions that can function as standards. You can then measure the glucose in your test solutions by comparing the color change in the test solution to the standards (make your best guess when the test color is in between two standards). For example, you could make standard solutions with 0, 0.25%, 0.5%, 1.0% and 2.0% glucose. The positive controls should bracket your test solution. So if the test solution (milk treated with lactase, for example) has 0.5% glucose, make sure your standard solutions have concentrations both above and below this concentration. The standard solutions can be made in water (or in glucose-free milk).
- How does the glucose concentration in regular milk change with time after you add the lactase? For example, what is the glucose concentration at 30 seconds, 1 minute, 2 minutes, and 5 minutes? Draw a graph of glucose concentration vs. time. When would you predict the rate of glucose production is fastest?
- Design an experiment where you determine how the reaction rate for glucose production depends on temperature. Vary the temperature, but keep everything else the same. What temperature would you predict is optimal for enzyme activity? At what temperature is the enzyme inactivated?
- Design an experiment where you determine how the enzyme reaction rate depends on pH.
Careers
If you like this project, you might enjoy exploring these related careers:
Contact Us
If you have purchased a kit for this project from Science Buddies, we are pleased to answer your questions.In your email, please follow these instructions:
- What is your Science Buddies kit order number?
- Please describe how you need help as thoroughly as possible:
Examples
Good Question I'm trying to do Experimental Procedure step #5, "Scrape the insulation from the wire. . ." How do I know when I've scraped enough?
Good Question I'm at Experimental Procedure step #7, "Move the magnet back and forth . . ." and the LED is not lighting up.
Bad Question I don't understand the instructions. Help!
Good Question I am purchasing my materials. Can I substitute a 1N34 diode for the 1N25 diode called for in the material list?
Bad Question Can I use a different part?
Contact Us