Abstract
You know that water can exist in three separate phases: solid (ice), liquid (water), and vapor (steam). To change from one phase to another, you simply add (or remove) heat. When water boils, what happens to molecules (for example sugar or salt) that are dissolved in the water? Do they boil off too, or do they stay behind?Summary
Objective
The goal of this project is to separate pure water from fruit juice using a simple stovetop distillation apparatus.Introduction
This project uses the technique of distillation. Distillation is when you boil a liquid, and then capture the vapor that escapes from the liquid and cool it. The cooled vapor condenses back into liquid. The condensed liquid is called the distillate. Do you think this process changes the liquid?
What if the liquid you boil has substances dissolved in it? For example, what if you started with a solution of sugar water? If you boiled the sugar water, you know from experience that there would be steam rising up from the pot on the stove. If you condensed that steam back into liquid, do you think the condensed liquid (the distillate) would contain sugar or not?
In this project, you will learn how to build a simple stove top distillation apparatus with stuff that you probably have in your kitchen right now. All you need is a deep pot with a sloping lid, a coffee cup, a bowl, some ice, and a stove. Of course, you'll also need a liquid to distill. Colored fruit juice will work fine, or you could make a solution of sugar water. Add food coloring to it if you like. The Experimental Procedure section, below, shows you how to put it all together to find out what happens.
Terms and Concepts
To do this project, you should do research that enables you to understand the following terms and concepts:- Boiling point
- Phases of matter:
- Solid
- Liquid
- Vapor
- Condensation
- Solvent
- Solute
- Distillate
Questions
- What happens to solute molecules when the solvent evaporates or boils?
- How will the distillate compare to the original juice for:
- color?
- taste?
- pH?
Bibliography
- For information on phase changes of water, see:
Nave, C.R. 2005. Phase Changes, HyperPhysics, Department of Physics and Astronomy, Georgia State University. Retrieved April 26, 2007. - Chamot, E. and B. Chamot, 2003. Molecular Level Motion of Different Phases, Chamot Laboratories, Inc. Retrieved April 26, 2007.
- For information on the water cycle, see: USGS, 2006. The Water Cycle, USGS Water Science Basics. Retrieved April 26, 2007.
Materials and Equipment
To do this experiment you will need the following materials and equipment:- Stove
- Deep cooking pot with sloped lid
- Ceramic coffee cup
- Ceramic bowl
- Ice
- Hot mitts
- Colored fruit juice (e.g., orange juice, grape juice, cranberry juice, etc.)
Experimental Procedure
- Do your background research so that you are familiar with the terms, concepts, and questions, above. For more information on distillation methods see Chemistry Lab Techniques.
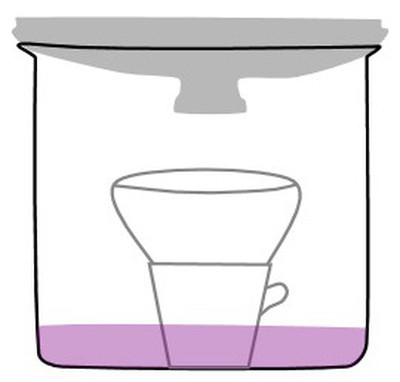
- The line drawing below is an illustration of the stove-top distillation apparatus used in this experiment.
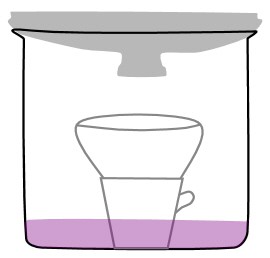
Line drawing of a stove top distillation apparatus. The text explains how to use it. - Here are the steps for using the distillation apparatus.
- Pour the colored fruit juice into the bottom of the pot. Save at least 200 ml of the original juice for comparison to the distillate.
- Place the ceramic coffee cup, open side up, in the center of the deep pot. (That's correct, right in the juice!)
- Place a bowl on top of the coffee cup. (The bowl will catch the condensed liquid that drips down from the lid.)
- Put the cover on the pot, upside down.
- Put ice in the cover of the pot. (You may have to replace the ice in the lid as it melts.)
- Turn on the burner to medium heat. You want the juice to boil moderately (not a rolling boil).
- Allow the pot to boil for 10 minutes or so (enough time to collect a sufficient amount of distillate for testing).
- When done, turn off the burner. Allow the pot to cool for a few minutes.
- Put on hot mitts and carefully remove the cover from the pot.
- Still wearing hot mitts, lift the bowl off of the coffee cup and set it down on a heat-resistant surface.
- Remove the coffee cup.
- After it cools, pour the remaining juice from the pot into a clear container.
- How do the original juice, the remaining juice from the pot, and the distillate compare in terms of color?
- Ordinarily in a chemistry experiment, you would not taste any of the solutions. In this case, since you are using clean kitchen utensils, and edible fruit juice, a taste test is OK. Let the liquids cool to room temperature before tasting them! How do the three different liquids compare for taste?
- Which liquid is sweetest?
- Which is least sweet?
- You should be able to explain why.
Ask an Expert
Variations
- In this experiment, you have three different liquids: the original liquid, the distillate, and the concentrated solution that is left over after boiling. Think of ways besides color and taste to compare the three liquids. For example:
- You could measure and use the conductivity of the original solution and distillate to compare various sports drinks and juices. Many claim that sports drinks help athletes because they are high in electrolytes, meaning they are electrically conductive. Are sports drinks really more conductive than juices? How does distillation affect conductivity of the liquids? Is there a certain brand of sports drink that is more conductive than others? For one method of measuring conductivity, see the Science Buddies project idea Electrolyte Challenge: Orange Juice Vs. Sports Drink.
- You could use pH paper to measure and compare the pH of each liquid.
- Pure water boils at 100°C at normal atmospheric pressure. As solute molecules are added to water, the boiling point increases. You could heat each of the liquids and measure their boiling points with a thermometer. Can you determine the concentration of solute molecules in each of the three liquids?
Careers
If you like this project, you might enjoy exploring these related careers:
Related Links
- Science Fair Project Guide
- Other Ideas Like This
- Chemistry Project Ideas
- My Favorites
- Chemistry Safety Guide