Abstract
Have you ever watched your parents dress up for a big evening? They may even splash on a bit of aftershave cologne or dab on some perfume since it is special occasion. But did you know that using perfume and scents is an ancient activity? Perfumes and the art of making perfume is an art that is thousands of years old. In this chemistry science fair project, you will learn more about one way to make perfume, called enfleurage, and experiment with it to extract your own floral scents.Summary
Michelle Maranowski, PhD, Science Buddies

Objective
To extract perfume oils from flowers using the enfleurage-extraction technique.
Introduction
Have you ever walked into the cosmetics section of a department store and smelled all of the different kinds of perfumes? Smelling can be kind of fun! Some perfumes are floral, some spicy, and some can even smell citrusy. It can be interesting to try to identify the different components that make up a typical perfume. But did you know that the art and science of making perfume is an ancient one that is about 4,000 years old?
The word perfume comes from Latin where per means through and fumum means smoke. Initially, perfumes were used in religious ceremonies, in the form of incense, but eventually, they were used by people to make themselves smell good. The Egyptian pharaohs used perfume. Perfumes were found in King Tutankhamen's tomb, and amazingly, the containers still had a fragrance when they were discovered, even though the liquid had evaporated away! The Greeks and Romans also delighted in perfume—wealthy Romans took baths in perfumed water. Until the 19th century, bad smells were thought to be the source of sickness and disease. During outbreaks of the bubonic plague, medieval doctors would protect themselves with masks and perfume. In the court of Louis XIV, King of France, bathing in water too often was thought to be unhealthy, so perfumes were used obsessively in order to mask the disagreeable smell in the royal palace.
Perfumes release their fragrances in three steps. The first step is the head note, which is the first impression that you get when you initially smell the fragrance. The heart note, the second step, forms the character of the perfume and is perceivable for 3–4 hours. The third step is the base note, which forms the foundation of the perfume and can last as long as a whole day. Fragrances can have a wide range of volatility. Volatility defines how a substance vaporizes. For instance, in a perfume made from lavender and vanilla fragrances, you will smell the lavender as part of the head note and the vanilla as part of the base note, since lavender has a higher volatility than vanilla. Most perfumes are complicated mixtures of several different synthetic or natural fragrances. In fact, many of the scents that are used to make up a perfume are synthetic. That is because extracting natural scents from flowers is time-consuming and expensive. It can take several pounds of raw plant material to extract just a few ounces of natural oil. There are also many natural sources for fragrances. For example, rose oil is extracted from the flower's petals, geranium oil is extracted from the plant's leaves, and cedar oil is extracted from grated cedar wood. Some fragrances have animal sources, like musk and ambergris.
There are several methods for extracting scents, such as distillation, maceration, expression, and enfleurage (AH-fluh-RAHJ). In distillation, the raw material is heated in a chamber and the fragrant compounds are recollected through condensation of the distilled vapor. In maceration, the fragrance is extracted by soaking the raw material in water, oil, or a solvent. The expression technique is a simple one. The raw materials are simply squeezed or pressed, and the oils are collected. Enfleurage is a two-step process during which the odor of aromatic materials is absorbed into wax or fat, and then extracted with alcohol.
In this chemistry science fair project, you will experiment with the enfleurage method of extraction. You will extract the fragrance from roses or lavender into vegetable shortening, and then use alcohol to extract the fragrance from the vegetable shortening. You will investigate how many rounds of enfleurage are required to yield a pleasant perfume, and then test your perfume using the noses of several volunteers. Let your noses do your work for you! Bet you never thought that chemistry could smell so good!
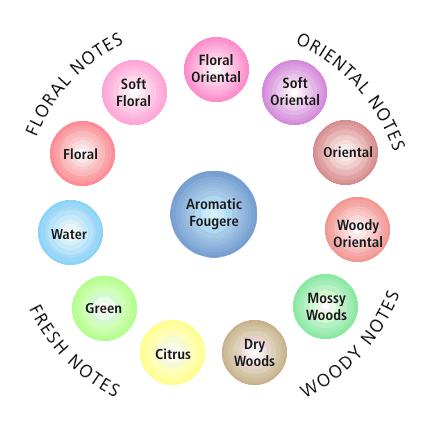 Image Credit: Wikipedia / Public domain
Image Credit: Wikipedia / Public domainA fragrance wheel is drawn with eleven circles in a ring with a single circle at its center. The top-right of the ring is categorized as oriental notes, the bottom-right is woody notes, the bottom-left is fresh notes and the top-left is floral notes. The circle at the center of the ring is labeled "Aromatic Fougere" and the circles around the ring, starting from the 12 o'clock position and moving clockwise are: floral oriental, soft oriental, oriental, woody oriental, mossy woods, dry woods, citrus, green, water, floral and soft floral.
Figure 1. The fragrance wheel was developed in 1983 by Michael Edwards and categorizes scents. (Wikipedia, 2009.)
Terms and Concepts
- Perfume
- Incense
- Volatility
- Vaporization
- Synthetic
- Musk
- Ambergris
- Distillation
- Maceration
- Solvent
- Expression
- Enfleurage
- Permeate
Questions
- What kinds of perfumes did the ancient Egyptians use? How did they make them?
- What method is used to extract lavender oil?
- Why is enfleurage used for some extractions, rather than the other methods?
- What is the world's costliest perfume, and what are some of the scents that are used to make it?
Bibliography
- Roach, J. (2007, March 29). Oldest Perfumes Found on Aphrodite's Island. Retrieved June 12, 2009.
- Parker, P. (n.d.). Using Shortening in Perfume Making. Retrieved June 12, 2009.
- Wikipedia Contributors. (2009, May 8). Fragrance Extraction. Wikipedia: The Free Encyclopedia. Retrieved June 12, 2009.
Materials and Equipment
- Flower petals, rose or lavender (9 cups)
- If you have access to fragrant roses or lavender from your yard, or from your neighbor's yard, feel free to use these (ask your neighbor's permission before picking the flowers).
- If you don't have free access to these flowers, go to your local florist. You will need to visit them every few days for fresh flowers.
- Just use the petals and avoid picking any other part of the flower.
- Plates, dinner-size (4)
- Scissors
- Cardboard (1 large piece); use a large cardboard box and cut pieces of cardboard from it, as needed
- Ruler
- Aluminum foil (1 roll)
- Knife or spreader
- Vegetable shortening (1 small container)
- Tablespoon
- Paper towels (1 roll)
- Table
- Books (at least 3). Each book should weigh about 5 pounds (lb.). If you don't have a 5-lb. book, you can use a couple of books that weigh 5 lbs. altogether.
- Watch
- Tweezers
- Measuring cup, liquid
- Ethyl alcohol (1 1/4 cup); available from your local pharmacy. It might be labeled as ethyl rubbing alcohol. Purchase alcohol that is 70% ethyl alcohol by volume. If you are not sure which is the right kind, ask the pharmacist.
- Paper coffee cup
- Double boiler
- If you don't have access to a double boiler, you can make your own using a stainless steel bowl that fits on top of a pot. The bowl should be a little larger than the pot so that it can rest just inside of it without touching the boiling water in the pot.
- Spoon
- Jelly jars and lids, small, cleaned in a dishwasher and completely air-dried (9)
- Masking tape
- Pen
- Sketchbook paper
- Volunteers, about the same age as you (3)
- Lab notebook
Experimental Procedure
Important Notes Before You Begin:
- This chemistry science fair project requires you to perform several different steps at the same time. Carefully read the entire procedure first, then plan accordingly.
- On the first day of the experiment, you will prepare three enfleurage sandwiches.
- On the second day, you will process one of the sandwiches into a perfume and replace the petals in the second and third sandwiches.
- On the third day, you will process the second sandwich into a perfume and replace the petals in the third sandwich.
- On the fourth day, you will process the third sandwich into a perfume and start your second trial.
- Be sure you water your flowers to keep them fresh as long as possible. When they start to wilt, you need to buy fresh flowers.
Performing the Experiment
- Pluck the flower petals off a few of the flowers and put them on the plate. You should have about 1 1/2 cups of flower petals.
- Using the scissors, cut out six pieces of cardboard that are each 4 inches x 4 inches.
- Take a piece of aluminum foil and wrap a piece of cardboard securely with the foil so that no cardboard is showing. Neatly fold the excess foil onto one side of the cardboard. Repeat this step with the five other pieces of cardboard.
- Spread about 1 tablespoon (tbsp.) of vegetable shortening onto one of the foil-covered cardboard squares with the knife or spreader. Do your best to spread it into a neat square that is no thicker than 1/4 inch. The square of shortening should be about 3 inches x 3 inches. If necessary, you can use more or less vegetable shortening. Repeat this step with the five other pieces of cardboard.
 Image Credit: Michelle Maranowski, Science Buddies / Science Buddies
Image Credit: Michelle Maranowski, Science Buddies / Science Buddies
Figure 2. Preparing for enfleurage.
- Roughly shred the flower petals with your hands if they are large. Take a 1/4 cup of petals and gently press them into the shortening on one of the cardboard pieces covered with shortening and foil. You should completely cover the shortening with the petals. Repeat this step with the rest of the cardboard pieces covered with shortening and foil.
 Image Credit: Michelle Maranowski, Science Buddies / Science Buddies
Image Credit: Michelle Maranowski, Science Buddies / Science Buddies
Figure 3. Foil-covered cardboard with vegetable shortening and rose petals.
- Put two pieces of covered cardboard together with the layer of shortening and petals in the middle, like a sandwich. Make two more sandwiches with the other covered cardboard pieces. You should now have three sandwiches of cardboard, foil, shortening, and petals.
- Rip off two paper towels, together, and fold them along the perforation. Put one of the sandwiches on the double sheet of paper towels and wrap the paper towel around it, flipping it over so the weight of the sandwich holds the folded-under pieces down. Repeat this step with the two additional sandwiches. You should now have three sandwiches, all for one trial.
- Find a location in your home that is free from drafts and will not be disturbed. Place the three sandwiches on a table and then place a 5-lb. book on each of the sandwiches. Your enfleurage setup is now complete. Note down the time and the date in your lab notebook.
- How long will it take for the scent of the flowers to permeate the shortening? What will happen to the petals? This is what you will find out. Let the sandwiches sit for one day (a full 24 hours), undisturbed.
- After one day, unwrap one of the sandwiches and gently pull apart the foil-covered cardboard pieces. What do the petals look like? Using the tweezers, pick off all of the petals from the shortening and discard them. How does the vegetable shortening smell? Note the time, the day, and all of your observations in your lab notebook. Clean the tweezers after using them.
- Using the knife or spreader, scrape and gather the vegetable shortening into one lump. You should have about 2 tbsp. of vegetable shortening. You will need to use an equivalent amount of alcohol. Put 2 tbsp. of alcohol in the paper cup.
- Read the instructions on how to use the double boiler, or use your homemade one. Put the appropriate amount of water into the bottom pot. If you are using a stainless steel bowl and a pot, instead of a double boiler, place some water in the bottom pot. Make sure that the water level is lower than the bottom of the bowl when it sits inside the larger pot. Place the bowl into the larger pot.
- Place the double boiler on the stovetop burner and turn on the burner to medium. Place the shortening in the upper pot. Slowly melt the vegetable shortening. Don't let the shortening boil or burn.
- When the vegetable shortening has completely melted, turn off the burner and add the ethyl alcohol to the shortening. Mix the alcohol and the vegetable shortening together well with the spoon. Allow the mixture to cool slightly.
- Once the mixture has cooled, pour it into a jelly jar and put the lid on. Using your masking tape and pen, write on the side of the jelly jar the trial and the days of enfleurage. Put the jar in a dark spot and let it sit for 1 full week. Discard the empty foil, but keep the cardboard pieces.
-
As soon as you have finished processing the first sandwich, obtain more flowers and remove the petals. Roughly shred the petals if they are large. You should have 1 cup of petals for this step.
- Unwrap the second sandwich and gently pull apart the foil-covered cardboard pieces. Using the tweezers, remove the petals from the vegetable shortening and discard them. Take a 1/4 cup of fresh petals and press them gently into the vegetable shortening of one of the foil-covered cardboard pieces. Take another 1/4 cup of fresh petals and gently press them into the vegetable shortening of the other foil-covered cardboard piece. Put the sandwich back together and rewrap it in the paper towel. Replace the sandwich under the book.
- Repeat this step with the third sandwich and the extra 1/2 cup of petals.
-
After another day (24 hours), obtain more flowers and remove the petals. Roughly shred the petals if they are large. You should have a 1/2 cup of petals for this step.
- Unwrap the second sandwich and repeat steps 10–15.
- Unwrap the third sandwich and repeat step 16, through bullet a, with the 1/2 cup of petals.
- After one more day, unwrap the third sandwich and repeat steps 10–15.
- On day 4, begin repeating the entire procedure, steps 1–18, two more times, for a total of three trials. Use fresh flowers and materials for each trial. Performing several trials ensures that your results are accurate and repeatable.
Analyzing the Results
- Cut the sketchbook paper into long, thin sections to make perfume testers. Make all of the testers the same size. They should be long enough that you can easily dip them into the jelly jars.
-
After the first trial of enfleurage perfumes has been sitting in the dark for at least a week (the first jar for 9 days, the second jar for 8 days and the third jar for 7 days), open the three jelly jars and compare the scents. Test one trial at a time.
- Label one end of each perfume tester with the trial and the number of enfleurage rounds.
- Dip the other end of the tester into the perfume. Tap the excess liquid off of the tester and place the tester on a plate with the wet end hanging off of the edge of the plate.
- Dip testers into the other two perfumes and place on the plate.
- Now smell the testers. Do you like the smell? Does it remind you of the scent of the flowers that you used? Which perfume do you like best? Remember to note down which enfleurage process you liked best in your lab notebook.
- Repeat this step with the other two trials.
- Repeat step 2 with your volunteers. Do your volunteers like the same scent that you do?
- Repeat steps 2–3 after the second trial has been sitting for at least a week.
- Repeat steps 2–3 after the third trial has been sitting for at least a week.
Ask an Expert
Variations
- In the chemistry science fair project described above, new petals were added to the vegetable shortening every day. How would the scent be affected if you let the petals sit for a longer time, like a week? Would the scent be pleasant? Try the experiment again and find out.
- Increase the amount of vegetable shortening used. Is there an optimal amount to use?
- Use butter in place of vegetable shortening. Is there a difference in the resulting scent?
- Try combining differently scented flowers in your enfleurage sandwiches to make a more complex scent
- Try another method for extracting scent from flowers, such as maceration. Try steeping petals in olive oil, almond oil, or jojoba oil.
Careers
If you like this project, you might enjoy exploring these related careers:
Related Links
- Science Fair Project Guide
- Other Ideas Like This
- Chemistry Project Ideas
- Cosmetic Chemistry Project Ideas
- My Favorites









