Paper Chromatography Resources
Log In
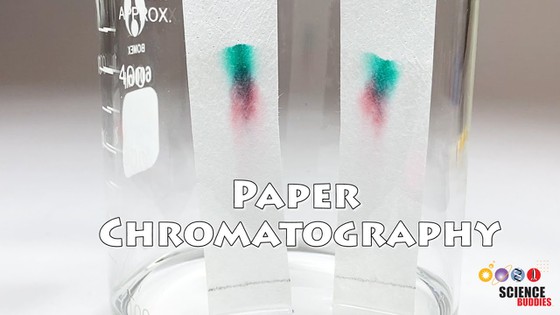
Paper chromatography is an inexpensive method for analyzing some types of chemical mixtures. A Candy Chromatography Science Kit is available to do several simple and fun paper chromatography experiments. You can learn more about paper chromatography in the video below. The video gives an overview of what paper chromatography is, shows how it is done, explains the separation processes involved, and also provides tips and tricks for troubleshooting your experiment.
If you would like to explore paper chromatography on your own here are our recommendations for suitable paper and readily available solvents for paper chromatography experiments at home.
Recommended Papers for Paper Chromatography
| Paper | Suitability | Comments |
| Laboratory filter paper | Excellent | - Inexpensive, yet gives excellent results. - Sometimes filter paper is available with porosities ranging from fine to coarse. We achieved good results with medium porosity paper, but others may work just as well. - Don't worry if all you can find is circular filter paper. Just cut strips from it. - Available from our partner Amazon.com. |
| Chromatography paper | Excellent | More expensive than laboratory filter paper. - Available from our partner Home Science Tools. |
| Paper towels (white) | OK | - Only the thick, sturdy paper towels work - Very porous which makes it difficult to get a nice "pattern." |
| Coffee filters (white) | OK | Absorbs solvent quickly, which makes it difficult to get a nice "pattern." |
| Printer / copier paper | Won't work | Does not absorb solvent. |
Readily Available Solvents for Paper Chromatography
You can find these solvents at grocery, drug, and /or hardware stores.
| Solvent | Polarity (arbitrary scale of 1-5) | Suitability | Comments |
| Water | 1 – Most polar | Good | |
| Rubbing alcohol (ethyl type) or denatured alcohol | 2 – High polarity | Good | POISONOUS! Primary ingredient is ethyl alcohol (ethanol), but it is mixed with other ingredients to make it poisonous. |
| Rubbing alcohol (isopropyl type) | 3 – Medium polarity | Good | POISONOUS! Primary ingredient is isopropyl alcohol (isopropanol). |
| Vinegar | 3 – Medium polarity | Good | |
| Nail polish remover (acetone) | 4 – Low polarity | Good | POISONOUS! USE OUTSIDE or with good ventilation. You might be able to find pure acetone at a hardware store. |
| Turpentine | 5 – Least polar | Good | POISONOUS! USE OUTSIDE or with good ventilation-it really smells bad. |
| Vegetable oil | 5 – Least polar | Poor | Too thick / viscous |
| Mineral oil | 5 – Least polar | Poor | Too thick / viscous |
Explore Our Science Videos
4 Easy Robot Science Projects for Kids
Make a Miniature Water Cycle Model
Can a Sandcastle Support a Brick?