Curl Metals With Heat!
Summary
Introduction
Do you like gift wrapping? If you do, you have probably curled a ribbon with scissors before. Have you ever wondered why the ribbon curls when you run a scissor blade down one side of it? The answer is that when you apply pressure on the ribbon with the blade, the outer layer of the ribbon stretches and expands. This makes the outside layer of the ribbon longer than the inside layer that is pressed against the blade. As a result, the ribbon starts to curl to make up for the different lengths of each of its layers. In this activity, you will also make materials curl. However, for these ones you won't need scissors; you will use heat instead!
Materials
- Aluminum foil
- Printer paper
- Scissors
- Glue
- Candle
- Lighter or matches
- Adult helper
- Optional: other material sheets (plastic, copper, etc.)
- Optional: blow dryer
 Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Prep Work
- Cut four half-inch wide strips of the aluminum foil.
 Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Image Credit: Svenja Lohner, Science Buddies / Science Buddies
- Cut four half-inch wide strips of the printer paper.
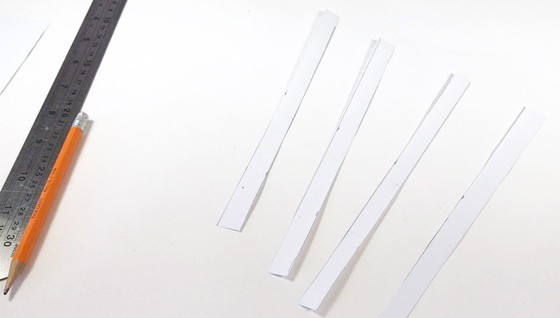 Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Image Credit: Svenja Lohner, Science Buddies / Science Buddies
- Glue two of the aluminum strips together, so there are two layers of aluminum on top of each other.
 Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Image Credit: Svenja Lohner, Science Buddies / Science Buddies
- Glue two of the paper strips together, so there are two layers of paper on top of each other.
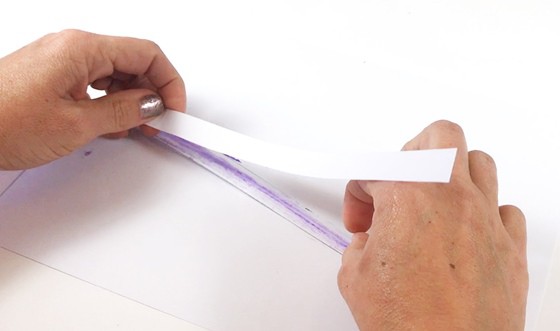 Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Image Credit: Svenja Lohner, Science Buddies / Science Buddies
- Glue one strip of paper on top of one strip of aluminum.
 Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Image Credit: Svenja Lohner, Science Buddies / Science Buddies
- Repeat the previous step with the remaining aluminum and paper strip.
- With the help of an adult, light the candle.
Instructions
- Take the strip that is made up of two layers of aluminum. With the help of an adult, carefully hold one end of the strip about 1.5-2 inches above the candle flame. Keep it there for about 3 seconds.What happens to the aluminum strip?
 Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Image Credit: Svenja Lohner, Science Buddies / Science Buddies
- Remove the aluminum strip from above the candle. Carefully touch the part of the strip that was above the flame.How does it feel compared to the rest of the strip?
- Take the strip that is made up of two layers of paper. Again, hold one end of the strip about 1.5-2 inches above the candle flame. Keep it there for 3 seconds, being careful to not accidentally burn the paper strip.What do you notice this time?
 Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Image Credit: Svenja Lohner, Science Buddies / Science Buddies
- Again, remove the paper strip from above the candle and gently touch the part of the strip that was above the flame.How does it feel?
- Next, take the strip that is made up of aluminum and paper. Hold one end of the strip above the candle flame as you did with the others. Have the aluminum layer face toward the candle and the paper layer toward the ceiling. Keep the strip above the candle for 3 seconds.What do you observe? Are your results different than before?
 Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Image Credit: Svenja Lohner, Science Buddies / Science Buddies
- After removing the strip from above the candle, touch the strip on both sides where it was above the flame.Do both sides feel the same?
- Finally, take the last strip made up of aluminum and paper. This time, hold the strip above the flame with the paper layer facing the candle flame and the aluminum layer facing up.What happens this time? Can you explain your results?
- Remove the strip from above the candle and touch it on both sides where it was above the flame.What do you notice?
Cleanup
- Make sure to blow out the candle, then dispose of your aluminum and paper strips in the trash.
What Happened?
Did you see one of your strips curl above the candle flame? You should have! The aluminum strip and the paper strip should have not changed when you held them above the flame. This is because the two layers of the strip are made of the same material. If the strip gets hot, which you should have noticed when touching it after removing it from above the flame, both layers expand exactly the same. However, if the strip consists of two different materials, such as the one you made from paper and aluminum, a change will happen.
When holding the paper-aluminum strip above the flame, you probably observed that it immediately started to bend or curl in one direction. It curls upwards when the aluminum layer faces the candle and downwards when the aluminum layer is on top. This is because both the aluminum and the paper layer heat up when above the flame. The aluminum expands more than the paper because of its higher thermal expansion coefficient. Because the paper and aluminum are glued together, the aluminum curls away from the paper strip to make up for the different lengths of each of its layers—exactly like the gift wrap ribbon! You will see the same results with other strips that consist of two different materials that have different thermal expansion coefficients. If you tried using a blow dryer for this experiment, you might have noticed that this was not a good idea. Although it also heats up the strips, it will also blow them away!
Digging Deeper
You probably know that materials are able to change their shape or volume when they are heated or cooled. This is true for solids, liquids, and gases, which are all made up of atoms and molecules. Once exposed to heat, these molecules begin to vibrate and move around very fast. This makes the molecules spread out and take up more space. As a result, a material expands when it gets hot. On the other hand, when it is cold, molecules move less which makes them take up less space. Thus, materials shrink when they get cold. Although all materials expand when heated, they do not expand to the same degree. How much a material expands when heated is described by its thermal expansion coefficient. For example, aluminum expands 21-24 micrometers per meter if you increase its temperature by 1 degree.
But what happens if an object is made up of more than one material? They will both expand differently when heated up! In fact, there are special materials, called bimetals, which make use of their different thermal expansion properties. A bimetal is an object that consists of two separate layers of two different metals that are joined together. When a bimetal is heated, one of the metals will expand more than the other. This results in the bimetal curving (or curling) in one direction just like the ribbon for the gift wrap. Because of this, bimetals are used to indicate temperature changes, such as in the pointer thermometers that you can find in fridges or offices. Inside these thermometers, a bimetal coil is attached to a pointer. When the temperature changes, the pointer moves depending on the deformation of the bimetal coil (see the video link in the Additional Resources section).
Ask an Expert
For Further Exploration
- What happens when you glue the aluminum strip to other materials such as a plastic or copper sheet? Do your results change?
- Does your experiment also work with other heat sources? Repeat the experiment with a blow dryer on a hot setting. Does it work? Why or why not?
Related Resources
Project Ideas
Links
- Scientific American: Secret of Ribbon Curling Revealed
- Wikipedia: BiMetal
- YouTube Video: A temperature measurement metallic spiral strip metal thermometer
- The Engineering ToolBox: Coefficients of Linear Thermal Expansion