Summary
Introduction
Do you know what enzymes are? They are like little machines that carry out many different chemical reactions that are essential for nature and our bodies. For example, they help break down the food we eat, or help make yogurt and cheese! In this activity, you will simulate an enzymatic reaction and explore what factors affect the activity of an enzyme. You only need a few simple items, including your hands, lots of toothpicks, and a timer.
Materials
- Toothpicks (about 150)
- Paper clips (50)
- Timer and stopwatch
- Paper
- Pencil
- Ice cubes
- Water
- Bowls (2)
- Optional: Helper
- Optional: Mitten or oven gloves
 Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Prep Work
- In this activity, you will use a toothpick model to simulate an enzymatic reaction. Your hand will represent the enzyme, the toothpicks are the enzyme's substrate, and the broken toothpicks are the reaction products. Breaking the toothpicks in half with your hand simulates an enzyme breaking down a substrate.
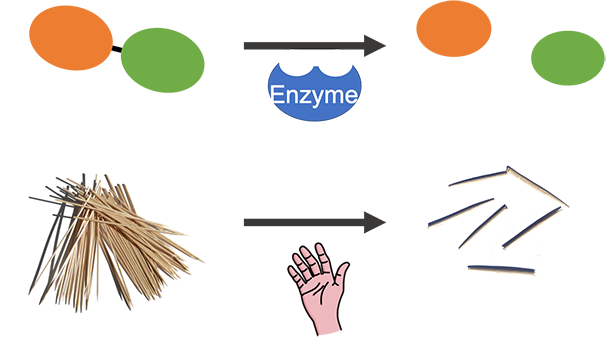 Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Image Credit: Svenja Lohner, Science Buddies / Science BuddiesThe above image shows two joint ellipses on the left that represent an enzyme's substrate. Next to the ellipses an arrow points to the right. A shape underneath the arrow represents the enzyme. Next to the arrow two separate ellipses are shown that represent the reaction products. The above image shows unbroken toothpicks on the left that in the toothpick model represent an enzyme's substrate. Next to the toothpicks an arrow points to the right. A hand underneath the arrow represents the enzyme in the toothpick model. Next to the arrow broken toothpicks are shown that represent the reaction products.
- To simulate an enzymatic reaction with the toothpick model, you have to follow these rules throughout your experiments.
- Only use one hand to break the toothpicks in half.
- Only break one toothpick at a time.
- Completely break each toothpick in half (bent toothpicks do not count).
- Only break each toothpick once.
- Put every broken toothpick back into the original bowl.
- Close your eyes while breaking the toothpicks.
How do you think do these rules reflect what is happening during a real enzymatic reaction? - Take 10 toothpicks and practice breaking them in half while following the rules stated in the previous step. Using your index finger and thumb might work best.
 Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Instructions
Experiment 1: Enzyme Activity Over Time
- You will simulate one continuous enzyme reaction and stop in between to look at your results so far. This means the whole reaction will be split into four rounds. On a sheet of paper, make a table like this:
Round Time in seconds Total number of broken toothpicks 1 5 2 10 3 20 4 40
- Place 50 unbroken toothpicks into a bowl.
- Round 1: Set a timer for 5 seconds.
- Start the timer, and immediately afterwards start breaking the toothpicks in the bowl with only one hand and your eyes closed. Break the toothpicks over the bowl you take them from so all the broken toothpicks will drop back into the same bowl. If you pick up a toothpick that is already broken, do not break it again, but drop it back into the bowl until you get an unbroken one.
 Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Image Credit: Svenja Lohner, Science Buddies / Science Buddies
- After 5 seconds, stop and count the number of broken toothpicks in the bowl. Write the results down in your table.
- Put all the broken and unbroken toothpicks back into the bowl.
- Round 2: Set the timer to 10 seconds.
- Again, start the timer and immediately afterwards start breaking the toothpicks in the bowl with only one hand and your eyes closed. Make sure to drop the broken toothpicks back into the bowl you took them from. Any already broken toothpicks that you pick up cannot be broken again. Drop these back into the bowl and pick up a new toothpick until you find an unbroken one.
Do you think you will be able to break more or fewer toothpicks than in the previous round?
- After 10 seconds, stop and count the number of broken toothpicks in the bowl. To determine the number of broken toothpicks you can also count the number of unbroken toothpicks and subtract them from the initial 50 toothpicks. Write the result in your data table.
- Put all broken and unbroken toothpicks back into the bowl and do two more rounds. In round 3, set the timer to 20 seconds and in round 4, set the timer to 40 seconds.
- Use the total number of broken toothpicks in your table to calculate how many toothpicks you broke in each round.
- Calculate the reaction rates (or enzyme activities) for each round. You can do this by dividing the number of toothpicks broken in that round by the number of seconds within that round. The result should tell you the reaction rate (or enzyme activity) in number of broken toothpicks per second. For example, if you broke 5 toothpicks in the first round, which was 5 seconds, then the reaction rate would be 5 toothpicks divided by 5 seconds, which is 1 toothpick per second.
- Compare the enzyme activities for each round.
What do you notice? What happens to the enzyme activity as the supply of unbroken toothpicks runs out over the course of the reaction?
Experiment 2: Effect of Substrate Concentration
- Make a table on a sheet of paper that shows the number of initial toothpicks in the bowl in one column and the number of broken toothpicks in the second column.
- Place 50 paper clips into an empty bowl.
What do you think the paper clips could represent?
- Round 1: Add 10 unbroken toothpicks to the paper clips in the bowl and mix them. This round will represent a low substrate concentration.
 Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Image Credit: Svenja Lohner, Science Buddies / Science Buddies
- Set the timer for 20 seconds.
- Start the timer and immediately afterwards start breaking the toothpicks in the bowl with only one hand and your eyes closed. Break the toothpicks over the bowl you take them from so all the broken toothpicks will drop back into the same bowl. If you pick up an already broken toothpick, do not break it again, but drop it back into the bowl until you get an unbroken one.
 Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Image Credit: Svenja Lohner, Science Buddies / Science Buddies
- After 20 seconds, stop and count the number of broken toothpicks in the bowl. Write the result in your data table.
Was it easy or difficult to find the toothpicks to break them in half?
- Empty the bowl.
- Repeat steps 2–7 for two more rounds, but change the number of initial toothpicks that you add to the paper clips each time. In round 2, add 25 toothpicks and in round 3, add 50 toothpicks to the paper clips in the bowl. Round 2 and 3 represent medium and high substrate concentrations, respectively.
- Look at your data table and compare your results for each round.
How did the enzyme activity change with the number of available toothpicks? What does your result tell you about the effect of substrate concentration on the reaction rate of enzymes?
Experiment 3: Effect of Temperature
- Fill a bowl with cold water and add some ice cubes.
- Place 20 toothpicks in an empty bowl.
- Start a stopwatch and try to break the 20 toothpicks as fast as you can. Remember that you can only break them with one hand and with your eyes closed. Break the toothpicks over the bowl you take them from so all the broken toothpicks will drop back into the same bowl. If you pick up an already broken toothpick, do not break it again, but drop it back into the bowl until you get an unbroken one.
 Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Image Credit: Svenja Lohner, Science Buddies / Science Buddies
- Once you have broken all 20 toothpicks, stop the stopwatch and write your time on a sheet of paper.
- Empty the bowl and put 20 new toothpicks inside.
- Place the hand you used to break the toothpicks in the prepared ice-cold water bath for 1 minute.
What do you think the ice bath simulates?
 Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Image Credit: Svenja Lohner, Science Buddies / Science Buddies
- Start the stopwatch and try to break the 20 toothpicks as fast as you can with your cold hand. Remember to follow all the rules!
- Stop the stopwatch once you have broken all the toothpicks and write down your time.
- Calculate the reaction rate for each round. Divide the number of broken toothpicks (20) by the time it took you to break them apart (in seconds). Compare the enzyme activities.
How did the enzyme reaction rate change with temperature? What does your result tell you about the effect of temperature on the enzyme activity?
Cleanup
What Happened?
As mentioned in the procedure, when breaking the toothpicks in half you simulated an enzymatic reaction. In this reaction, the hand breaking the toothpick represented the enzyme, with the thumb and the index finger being the active site of the enzyme. The toothpick represented the substrate that is broken down due to the enzymatic activity. The broken toothpick represents the products of the enzyme reaction. Closing your eyes mimicked the randomness with which an enzyme reacts with its substrate, and just like products and reactants continue to mix during a chemical reaction, you were mixing the broken and unbroken toothpicks inside the bowl. In Experiment 1 you should have noticed that the enzymatic activity decreased over the course of the reaction. This can be explained by the fact that the substrate concentration (number of unbroken toothpicks) continuously decreased over time and it was more difficult for the enzyme to find available substrate (unbroken toothpicks) to react with. With increasing reaction time, you probably were picking up a lot of toothpicks that were already broken and it might have taken a while to find a toothpick that was still intact.
Experiment 2 demonstrated the effect of substrate concentration on enzyme activity in a different way. The paper clips in this experiment represented a solvent that the substrate is dissolved in. In every round, you increased the substrate concentration or number of unbroken toothpicks in the solvent. You should have noticed that the enzymatic activity or the enzyme's reaction rate increased with increasing substrate concentration as there was more substrate available for the enzyme to react with. You might not have seen this in your experiment, but this reaction rate increase is only true initially. As the substrate concentration increases more and more, the enzyme eventually becomes saturated, which means that there is more substrate around than the available enzyme can bind. This means at some point the enzyme activity will not increase anymore, even if you add more substrate.
In Experiment 3, you should have noticed that temperature does have a significant effect on enzyme activity. Usually, the enzyme activity decreases with decreasing temperature. This happens because molecules move much slower in a cold environment than in a hot environment. As the substrate and enzyme molecules move slower, the chances of an enzyme molecule colliding with and interacting with a substrate molecule are much lower. As a result, the reaction rate decreases.
If you did the experiments in the Further Exploration section, you should have observed that increasing the enzyme concentration (adding a second hand breaking toothpicks) increases the reaction rate of the enzyme. You were also able to see that enzymes can be inhibited by external factors such as chemicals (in your experiment, represented by a bulky glove). In the presence of inhibitors, the reaction rate of an enzyme decreases significantly.
Digging Deeper
Enzymes are essential for life, and play an important role in all the chemical reactions that happen within our bodies. They are proteins made by our cells that act as biological catalysts and increase the rate of a reaction that otherwise might not happen or would take too long to be beneficial. Enzymes are able to speed up a chemical reaction by reducing its activation energy while interacting with its reactants. Every enzyme has an active site, which is where the reaction takes place. These sites are like special pockets that are able to bind a molecule. The enzyme pocket has a unique shape so that only one specific substrate (target molecule) is able to bind to it (Figure 1). This means that enzymes are usually highly specific for a particular chemical reaction. Once the molecule is bound to the enzyme, the chemical reaction takes place. Then, the reaction products are released from the pocket and the enzyme is ready to start all over again with another substrate molecule.
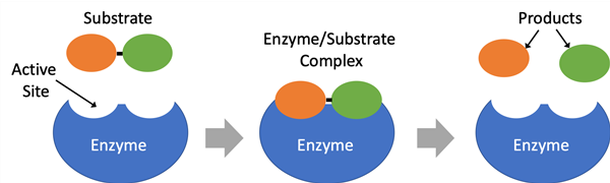 Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Image Credit: Svenja Lohner, Science Buddies / Science BuddiesThe enzyme binds its substrate at the active site to form an enzyme/substrate complex. Once the reaction is completed, the reaction products are released from the active site of the enzyme.
Figure 1. Schematic drawing of an enzyme reacting with its substrate.
An enzyme's activity tells you how well an enzyme performs its function and how fast the reaction takes place. The study of how enzymes change the rate at which a chemical reaction occurs is called enzyme kinetics. Enzymatic activity, or the reaction rate of the enzymatic reaction, is usually measured by doing an enzyme assay. An enzyme assay measures either the disappearance of the substrates or the appearance of products over time (Figure 2). It is also possible to measure other variables that are a proxy for either of those. The rate at which the substrates disappear, or the products appear, is a measure of the enzyme's activity.
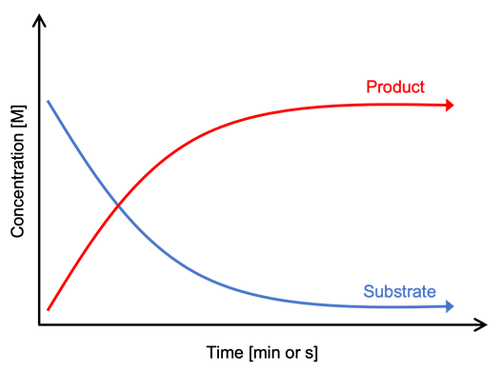 Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Image Credit: Svenja Lohner, Science Buddies / Science BuddiesAs the reaction takes place, the amount of substrate decreases whereas the amount of product increases.
Figure 2. Example graph showing the appearing products or disappearing substrates during an enzyme assay. As the reaction takes place, the amount of substrate decreases, and the amount of product increases.
Ask an Expert
For Further Exploration
- Besides substrate concentration and temperature, there are other factors that change an enzyme's reaction rate. One of these is the enzyme concentration in the reaction. Change the enzyme concentration in your toothpick reaction by adding more enzyme. Ask a volunteer to help you break the toothpicks. Make sure he or she follows the same rules as you are! As you add more enzyme (or hands) to the mix, how does the reaction rate change?
- Enzymes can also be inhibited or activated using chemicals or other factors that act as inhibitors or activators. Find a bulky glove, such as a mitten or oven mitt, and put in on the hand you will use to break the toothpicks. Then break 20 toothpicks as fast as you can with and without the glove on. Does the glove act as an enzyme inhibitor or activator?
- Make a graph for your first experiment, with the total reaction time (in seconds) on the x-axis and the total number of broken toothpicks on the y-axis. You will need to add the times for each round to get the total reaction time. What does the graph tell you about the enzyme activity over time?
Related Resources
Project Ideas
Activities
Lesson Plans
- 5-PS1-4. Conduct an investigation to determine whether the mixing of two or more substances results in new substances.
- HS-PS1-5. Apply scientific principles and evidence to provide an explanation about the effects of changing the temperature or concentration of the reacting particles on the rate at which a reaction occurs.