E-factor: Environmental Impact Factor for Chemical Reactions
Summary
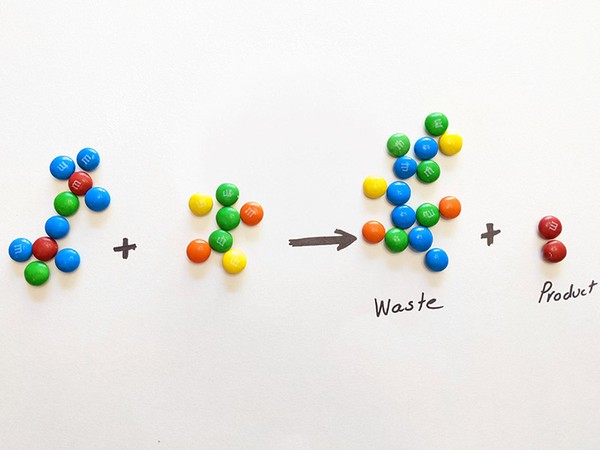
Overview
In this lesson, students will do a simple exercise with M&Ms to understand what environmental impact factor (E-factor) is, how it applies to chemical processes, and how waste from chemical reactions can be reduced by applying the principles of green chemistry.
Learning Objectives
Students will:
- Understand strategies for reducing waste
- Perform an exercise which has them practice E-factor
- Relate the exercise to chemical processes
NGSS Alignment
- MS-PS1-3. Gather and make sense of information to describe that synthetic materials come from natural resources and impact society.
- HS-ETS1-1. Analyze a major global challenge to specify qualitative and quantitative criteria and constraints for solutions that account for societal needs and wants.
- HS-ETS1-2. Design a solution to a complex real-world problem by breaking it down into smaller, more manageable problems that can be solved through engineering.
- HS-ETS1-3. Evaluate a solution to a complex real-world problem based on prioritized criteria and trade-offs that account for a range of constraints, including cost, safety, reliability, and aesthetics, as well as possible social, cultural, and environmental impacts.
Materials
- 15 small bags of M&Ms
- 15 balances or digital scales
- 30 cups
- 15 calculators
- Optional: plastic gloves
- Bulk bag of M&Ms
- 5 sealable plastic bags
- Student worksheet
Background Information for Teachers
The environmental impact factor (E-factor) relates to the 2nd Principle of Green Chemistry, Atom Economy. Frequently applied in an industrial sense, the E-Factor compares the amount of useful product to the amount of waste produced in a chemical process. It is calculated using the simple equation:
For more complex products, it is common to have a manufacturing process that has several chemical reactions. If each chemical reaction produces waste the resulting E-factor can be quite high.
The principles of Green Chemistry can be used to diminish the amount of waste created and lower the environmental impact factor of a particular chemical process. Watch the video to see what Green Chemistry is.
Green Chemistry Principles Addressed
- Prevention: Design chemical syntheses to prevent waste, leaving no waste to treat or clean up.
- Safer Solvents & Auxiliaries: Avoid using solvents, separation agents, or other auxiliary chemicals. If these chemicals are necessary, use innocuous chemicals.
- Use of Renewable Feedstocks: Use raw materials and feedstocks that are renewable. Renewable feedstocks = farm products or the wastes of other processes; depleting feedstocks = fossil fuels (petroleum, natural gas, or coal) or are mined.
- Reduce Derivatives: Avoid using blocking or protecting groups or any temporary modifications if possible. Derivatives use additional reagents and generate waste.
- Catalysis: Minimize waste by using catalytic reactions. Catalysts are used in small amounts and can carry out a single reaction many times. They are preferable to stoichiometric reagents, which are used in excess and work only once.
- Real-time Analysis for Pollution Prevention: Include in-process real-time monitoring and control during syntheses to minimize or eliminate the formation of byproducts.
Prep work
- Prepare five baggies of M&Ms as shown below.
| E-factor | M&M model | Industry segment |
|---|---|---|
| 0.1 |
 Image Credit: Science Buddies Image Credit: Science Buddies
|
Petrochemicals A chemical derived from Petroleum or natural gas Example: Solvents detergents, adhesives |
| 1 |
 Image Credit: Science Buddies Image Credit: Science Buddies
|
Bulk Chemicals plastics and polymers Example: plastic bottles, grocery bags |
| 10 |
 Image Credit: Science Buddies Image Credit: Science Buddies
|
|
| 100 |
 Image Credit: Science Buddies Image Credit: Science Buddies
|
Fine Chemicals Chemicals used to make specific items Example: coating on laptop screens, electronics parts |
| 250 |
 Image Credit: Science Buddies Image Credit: Science Buddies
|
Pharmaceuticals Example: antibiotics, blood thinners |
Procedure
- Show students the accompanying PowerPoint slides to explain E-factor and how it relates to a manufacturing process.
- Stop at slide 5!
- Ask students to get into groups of two or three.
- Give student worksheets to each group.
- Hand out a small bag of M&Ms to each group of students.
- Explain that this bag of M&Ms is very special but that unfortunately the only ones that you can use are the red ones.
- Following the directions on the student sheet, have students calculate the E-factor and answer the questions.
- Allow students to share their E-factor.
- Move onto slide 6, the challenge of using the 12 principles of green chemistry to reduce the E-factor.
- Tell students they must come up with specific strategies of how they can reduce their waste in the lab.
- Move around the room and place a star by viable strategies that students have brainstormed.
- Have students share their ideas for waste reduction.
- Share next slides. Explain to students that for each strategy they have written down, they can remove one color from their waste pile.
- Remind students that this only applies to strategies you have approved!
- Using their new product, have students recalculate their E-factor.
- Have students throw away any M&Ms left in their waste pile. They may eat their product and strategy M&Ms.
- Show students the prepared bags of M&Ms and explain how these bags represent chemical processes.
- Ask students if any of them use Ibuprofen. You may have to prompt them with over-the-counter brand names of the drug.
- Project the slide of the Ibuprofen.
- Explain that at the time Ibuprofen was invented it was a big breakthrough for people suffering from joint and muscle pain, it was originally invented to help arthritis patients. Sounds great right? Until you look at how much of the process created waste.
- With the old method, that means that there would be 68.72 tons of waste = 69 female walruses of waste.
- The process was changed in 1990 through the use of green chemistry techniques.
- Show the next slide with the new process. Ask the students to tell you what they immediately see as being different.
- Talk the students through the new process. Point out that the acetic acid is used as a catalyst.
- Point out that the current and better method = 13.33 tons of waste, 13 female walruses.
- In the new method, a mere 1% of the building block atoms result as waste. The new process also replaced a six-step with a three-step process, aiding energy efficiency (principle 6) and simplifying real-time analysis for pollution prevention (principle 11).
- Previously the waste went mostly to the landfill. Today, scientists look at the by-products too and think about how those by-products can be used to make something else rather than be discarded. It is just like when you have a Thanksgiving turkey. Try to imagine all the various foods you can make out of that one turkey.
Assess
You can collect and review, using the answer key, the students' worksheets as a way of assessing whether students understood the lesson.