Why Won't it Mix? Discover the Brazil Nut Effect
Summary
Introduction
Have you ever noticed that the dried fruits or nuts in your breakfast cereal are not evenly spread out inside the box, or that in a container of mixed nuts Brazil nuts gather at the top? This phenomenon is commonly called the "Brazil nut effect," and the science behind it is surprisingly complex and far-reaching. This phenomenon can be a nuisance when you want to fill silos, bags, or bins with different types of materials. It can also be used to our advantage: an avalanche airbag uses the Brazil nut effect to keep skiers on top of the snow during an avalanche! In this activity, you challenge yourself to mix different kinds of granular materials. It's not as easy as it sounds!
Materials
- Empty, transparent cylindrical container. Electrical tape containers, makeup containers, or Petri dishes are good options. A small food container works too.
- Colored sand (available at arts and crafts stores), enough to fill your container 1/3 full. Sifted dry sand from a sandbox or extra fine granular sugar is fine, too.
- Single color of dessert sprinkles. Make sure your sand and sprinkles are each a different color so when mixed, you can easily tell them apart.
- Measuring spoon
- Small spoon or spatula
- Additional granular materials such as lentils, flour, rice, ground coffee or coffee beans, dried fruit, etcetera
 Image Credit: Sabine De Brabandere, Science Buddies / Science Buddies
Image Credit: Sabine De Brabandere, Science Buddies / Science Buddies
Instructions
- Add a measuring spoon of sprinkles to your container, followed by a spoonful of sand. Continue until your container is about one half full with the sand-sprinkle mixture. Close the container. If needed, add tape around any tiny openings to prevent sand from seeping out.Are the sand and sprinkles in your container well mixed? What do you think will happen if you shake your container up and down, or from side to side?
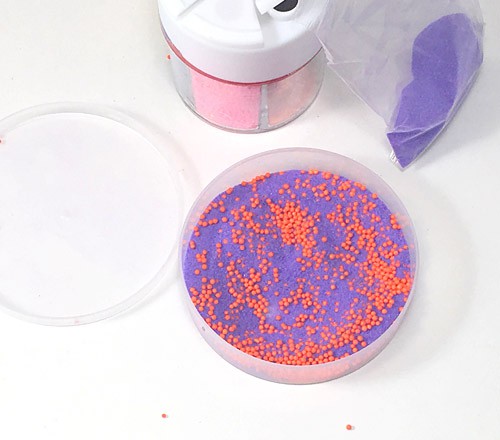 Image Credit: Sabine De Brabandere, Science Buddies / Science Buddies
Image Credit: Sabine De Brabandere, Science Buddies / Science Buddies
- Move your container up and down, first slowly, and gradually faster. Observe what happens.When do the sand and sprinkles start moving inside the box?
- If the sand collects in one part of the container, and sprinkles collect in another, we say they separate. If they are both found everywhere in the box, we say the mixture is well mixed.Do the sand and sprinkles separate when you shake the container? Why or why not?
- Open your container and use a small spoon or spatula to mix the sprinkles into the sand. Close your container and reapply tape if needed.
- Move your container from side to side, first slowly, and gradually faster. Observe what happens.At what point do the sand and sprinkles start to move inside the box? Do the sand and sprinkles separate when you shake the container slowly? Do they separate when you shake the container vigorously? Why do you think this happens?
- Open your container and use a small spoon to mix the sprinkles into the sand. Close your container and reapply tape if needed.
- Next, you will rotate your container. You could roll it from one side of a table to the other, or roll it away from you on the ground.Do you think the mixture will stay well mixed, or mix better by this movement?
 Image Credit: Sabine De Brabandere, Science Buddies / Science Buddies
Image Credit: Sabine De Brabandere, Science Buddies / Science Buddies
- Try it out!What do you observe? Why do you think this happens?
- Move your container in whatever way you like.Can you move your container in a way that mixes your sprinkles into the sand?
- Take a new cylindrical container or reuse container you just used, fill it about 1/2 full with a different mixture of granular materials and repeat step 9.Can you move your container in a way that mixes these granular materials? Why do you think this happens?
What Happened?
Regardless of the way you move your container, as soon as you moved it vigorously enough, the granular materials probably separated. This is expected.
Sand grains are much smaller than sprinkles, and they might be denser, too. If you shake the box vigorously enough, the particles of sand and sprinkles inside start to jump up or glide alongside and over each other. The small sand grains fall through small cracks between sprinkles, so the large sprinkles aggregate at the top and sides of your container. As soon as the individual particles of the granular materials you try to mix are different enough, moving the container does not help to mix them.
The separation and patterns are pretty, but this separation can be annoying when you want your muesli, trail mix, or nuts to stay mixed!
 Image Credit: Sabine De Brabandere, Science Buddies / Science Buddies
Image Credit: Sabine De Brabandere, Science Buddies / Science Buddies
Digging Deeper
We frequently encounter three states of matter: solids, liquids, and gases. We know that rocks are solid, the water in the ocean is liquid, and the air we breathe is gas. The classification is not always that clear when it comes to the way some materials behave. You can pile sand on sand like a solid, but you can also pour it so it flows more like a liquid. This is because it is a granular material, which is made up of many individual particles. Each individual particle is called a grain.
Granular materials can range in size from tiny powders like sugar and flour, to very large objects like rocks and boulders. In order for a granular material to show characteristics of a liquid there must be many grains close together.
For example, a couple of boulders rolling down a hill are not acting like a liquid, but thousands of boulders flowing down a hill during a landslide are. Similarly, a single piece of cereal or a single grain of sand does not act like a liquid, but your cereal can flow more like a liquid when you pour a bunch of it into a bowl or sand can flow more like a liquid when you pour a bunch of it out of a pail.
To mix different granular materials, you might think of shaking the container, or rotating it like in a tumbler. Unfortunately, these motions can induce separation. They make the grains jump up or roll over each other. As smaller grains fall through the spaces between larger grains, large particles tend to move toward the top in a process called percolation. Buoyancy also plays a role: buoyancy makes denser grains sink, while less dense grains float to the top. Another contributing factor is granular convection, or the phenomenon that granular materials move in convection-like circulation patterns when they vibrate. The larger, denser pieces follow the circulation pattern up, but fail to move down. Even the air between the particles and the container's shape are believed to make an impact.
Ask an Expert
For Further Exploration
- Make trail mix. Start with two ingredients, such as peanuts and raisins, and shake the container. How well do these mix? Add a third and then a fourth ingredient, such as sunflower seeds and freeze-dried strawberries. How well do all of the ingredients mix when shaken? What about rolled or poured? Try different ingredients. Which combinations mix well? Do more ingredients make a better-mixed trail mix?
- Make popcorn in a pot. Notice how you shake the pot while the popcorn pops. Observe the results. Do larger pieces or smaller ones end up on top? Where can you find the denser unpopped kernels? Do you see a similar distribution when popping corn in a microwave? Why would it be different?
- Try the activity with two types of sand: black coarser sand and pink finer sand. Will these be different enough to separate?
Related Resources
Project Ideas
Activities
Links
- Why brazils always end up on top, from BBC news
- The Brazil Nut Effect, by fyfluiddynamics
- How to Survive an Avalanche: Skier's Air Bag, from Scientific American