Abstract
Jumping discs can be a fun toy to play with, and with their sudden POP!, they can even be a good way to startle people who have never heard them before. Jumping discs use a neat trick to jump. They are made of two different types of metal, and these metals expand when they heat up (or shrink when they cool down), but not by exactly the same amount. In this science project you will explore how temperature affects the reactions of your jumping discs— and how to get the timing right if you want to make someone jump!Summary
Do not use the jumping discs on surfaces that are fragile or can be easily scratched.
Objective
Measure the effect of surface temperature on how long it takes a jumping disc to jump.Introduction
If you have seen a jumping disc, you might think it was nothing but a simple toy. How could you possibly use one for a science experiment? It turns out that jumping discs use some pretty neat tricks to work that depend on certain properties of materials. How they work actually depends on temperature. So in this project, you will examine how the temperature of the surface they are on changes how long it takes them to jump. If you have not seen a metal jumping disc before, watch this video before you continue:
The first trick jumping discs rely on is that most materials expand a little bit when they heat up, and shrink a little bit when they cool down (see Figure 1). This is called thermal expansion ("thermal" means having to do with heat or cold). Note that engineers usually just say "thermal expansion" to save time, instead of "thermal expansion and contraction". We tend not to notice thermal expansion much in everyday life (you have probably never noticed yourself getting taller when you come inside on a cold day, have you?). Normally things only expand or contract by tiny amounts. However, just because we do not usually notice thermal expansion does not mean that it is not important. Engineers have to think about thermal expansion when designing large things like roads, bridges, buildings, and railroad tracks (search the Web for images of "thermal expansion railroad tracks" — what do you see?). Thermal expansion can also be useful — have you ever seen a thermometer with liquid inside? Expansion and contraction of the liquid as it heats and cools can be used to measure temperature.
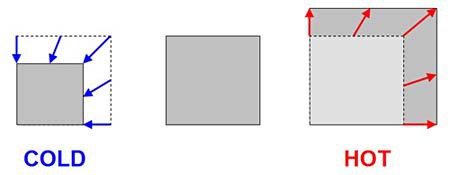 Image Credit: Ben Finio, Science Buddies / Science Buddies
Image Credit: Ben Finio, Science Buddies / Science Buddies
Figure 1. Thermal expansion (and contraction) means that most materials expand when they get hot and shrink when they get cold. Normally the amount of expansion or contraction is very small — we have exaggerated it here to make it easier to see.
The second trick about jumping discs is that they are bimetallic, meaning they are made out of two different kinds of metal (the prefix "bi" means "two"). These two metals undergo different amounts of thermal expansion— so when they are stuck together and heated up, they bend (see Figure 2 for an illustration). This concept is used in applications like the circuit breaker in your home. A circuit breaker uses a bimetallic strip with electricity running through it. When too much electricity runs through the strip, it gets hot and bends — "breaking" the circuit and stopping electricity from flowing. This is a safety measure to prevent the wires in your home from getting so hot that they catch on fire!
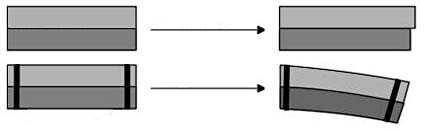 Image Credit: Wikimedia Commons / Creative Commons Attribution-Share Alike 3.0 Unported
Image Credit: Wikimedia Commons / Creative Commons Attribution-Share Alike 3.0 Unported
Figure 2. A bimetallic strip is made of two types of metal (light and dark gray in the picture) with two different degrees of thermal expansion. If the metals are not bonded together, then they will freely expand by different amounts — in this figure, the light gray strip expands more than the dark gray strip (top row). However, if the metals are bonded together (as shown by the black bars), they cannot expand freely. The light gray strip still expands more — forcing the entire strip to bend (bottom row). (Wikimedia Commons user Bemoeial, 2004)
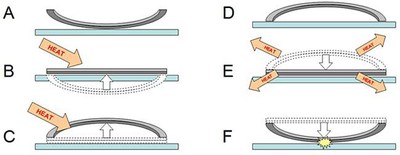
Bimetallic jumping discs work similarly to bimetallic strips, except they are circle-shaped. Jumping discs do not come perfectly flat, however — they are slightly bowed in one direction. When they are heated past a certain temperature, the jumping discs "snap through" in the other direction. Then, as they cool down, they snap back through to their original position — and this happens very quickly, which is what makes the disc jump! This process is shown in steps A through F in Figure 3.
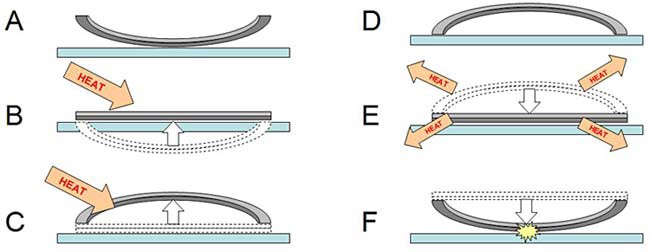
Diagram showing a cross-section of a metal disc that is made of two different metal sheets pressed together (shown in light and dark grey). When heat is applied, the light grey metal expands more than the dark grey metal, causing the disk to bend. When the disk is allowed to cool, the light grey metal shrinks more than the dark grey metal causing the disc to jump.
Figure 3. This figure shows a cross section of the jumping disc with its curve exaggerated to make the jumping process easier to see. The two metal layers of the disc are shown in two different colors (light and dark gray). (A) The disc starts out resting on a tabletop with its edges curved upward. (B) Once you apply heat, both layers of metal start expanding. However, the light gray layer expands more than the dark gray layer, forcing the disc to bend in the other direction. (C) After you add enough heat, the disc "snaps through" — its edges now curl downward. (D) The disc is now bent in the opposite direction from step A — it is "loaded" and ready to jump! (E) The disc is hotter than its surroundings, so it will start losing heat to the surrounding air and the tabletop. As the metals cool, the light gray metal shrinks more than the dark gray metal, causing the disc to start bending back to its original position. (F) Eventually the disc reaches a critical point and snaps through quickly — causing it to hit the table and jump with a pop!
The process in Figure 3 relies on heat transfer. Heat transfer is when heat moves from something warmer to something colder. Heat transfer can happen when two objects physically touch each other, which is called conduction. Heat can also be transferred through the air between two objects that are not touching, which is called convection. Heat can even be transferred through the vacuum of space! This is called radiant heat transfer and it is how the sun warms the earth. You may have noticed conduction, convection, and radiant heat transfer in everyday life. For example, if you pick up an ice cube, it will feel cold because heat conducts from your hand to the ice cube. If you pick up a warm rock that has been sitting in the sun, it will feel warm because heat conducts from the rock to your hand. If you stand near a heater in your house, you will feel hot air due to convection even if you do not actually touch the heater (and the same would be true for an air conditioner and cold air). And of course, if you stand outside on a sunny day, your skin will feel warm due to radiant heat transfer from the sun (Note: the effects of conduction, convection and radiant heat transfer add up, and can even cancel each other out. For example, if you stand outside on a cold, sunny winter day — your skin may feel cold, because the cold air convects heat away from your skin faster than the sun warms it).
Heat transfer happens faster when the temperature difference between two things is large, and it does not happen at all when two things are the same temperature (as shown in Figure 4). Because heat moves from hot to cold, two things that start at different temperatures will reach the same temperature eventually. You may have noticed this if you have ever left a hot drink (like the cup of hot chocolate in Figure 5) on the counter — eventually it will cool down and reach room temperature (usually about 70 degrees Fahrenheit or 21 degrees Celsius). This occurs because heat transfers from the hot drink to the surrounding room, which is colder. The opposite happens if you leave a cold object (like the carton of ice cream in Figure 5) on the counter — it will get warmer until it reaches room temperature, because heat is transferred from the warmer room to the colder drink, as shown in Figure 5.
Technical Note
The process whereby heat always flows from hot to cold is described by the second law of thermodynamics. The second law of thermodynamics states that it is impossible for heat to flow from cold to hot on its own without using additional energy. This is why air conditioners must use an added energy source like electricity — you cannot just cool down your house for free!
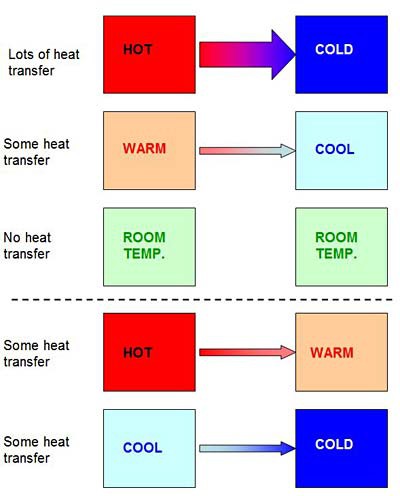 Image Credit: Ben Finio, Science Buddies / Science Buddies
Image Credit: Ben Finio, Science Buddies / Science BuddiesA diagram shows how heat is transferred between two objects of different temperatures. Heat transfer always moves from hot to cold and more heat transfer occurs between objects with a greater difference in temperature (i.e. Hot to Cold) than between objects with smaller differentials in temperature (i.e. Hot to Warm or Cool to Cold).
Figure 4. The basic law of heat transfer is that heat always moves from hotter to colder. This happens faster if the temperature difference between two things is large (one of them is very hot or very cold), and slower if the temperature difference is not as big. If two things are the same temperature, they will not transfer heat at all. Important: heat transfer can still occur between two things that are both warmer or both cooler than room temperature. For example, a glass of cold water will transfer heat to a frozen ice cube — even though they are both already below room temperature.
 Image Credit: Ben Finio, Science Buddies / Science Buddies
Image Credit: Ben Finio, Science Buddies / Science Buddies
Figure 5. Heat transfer on a kitchen countertop. Heat flows out of the hot chocolate into the countertop (by conduction) and surrounding air (by convection), and into the cold ice cream. Eventually both the hot chocolate and the ice cream will reach room temperature. We drew arrows on the picture because heat transfer is invisible — you can't actually see it happening (although you can observe the effect it has over time — for example, the ice cream will begin to melt).
This means that how fast your jumping disc jumps depends on how hot (or cold) its surroundings are. In part E of Figure 3, the jumping disc is hot and its surroundings are at room temperature. In this experiment you will figure out exactly how the temperature of the surroundings affects your jumping disc. Can you figure out what will happen if the surroundings are hotter or colder than room temperature? That will enable you to time your jumping disc's pop perfectly if you want to startle someone!
Terms and Concepts
- Thermal expansion
- Bimetallic
- Circuit breaker
- Heat transfer
- Conduction
- Convection
- Radiant heat transfer
- Room temperature
- Second law of thermodynamics
Questions
- Can you find some real-life examples of thermal expansion?
- What other uses do bimetallic strips have besides circuit breakers?
- Where else do you see heat transfer in everyday life? (Hint: think about cooking.)
- Can you think of different examples of conduction, convection and radiant heat transfer that you experience every day?
- How much do you think the temperature of the tabletop will affect how long it takes your jumping disc to jump? What will happen if the tabletop is hot? What will happen if it is cold?
Bibliography
The following references may help you explore more about heat transfer, bimetallic strips, and the jumping disc.
- The Physics Classroom. (n.d.). Introduction to thermal physics. Retrieved January 15, 2013.
- Rader, A. (n.d.). Thermodynamics. Physics4Kids. Retrieved February 27, 2013.
- Wikipedia contributors (2012, November 19). Bimetallic strip. Retrieved January 15, 2013.
For help creating graphs, try this website:
- National Center for Education Statistics, (n.d.). Create a Graph. Retrieved June 25, 2020.
Materials and Equipment
- Bimetallic jumping discs, available online from hobby and educational supply stores. You may need to search for a site that will ship to your area.
- Hair dryer (heat source for the discs and countertop)
- Ice cubes and plastic bag, or a blue ice pack (something else from your freezer will work - like a bag of frozen vegetables, but remember that re-freezing defrosted vegetables is not recommended)
- Stopwatch
- Paper towels or dish towel
- Hard counter or table surface
- Lab notebook
Disclaimer: Science Buddies participates in affiliate programs with Home Science Tools, Amazon.com, Carolina Biological, and Jameco Electronics. Proceeds from the affiliate programs help support Science Buddies, a 501(c)(3) public charity, and keep our resources free for everyone. Our top priority is student learning. If you have any comments (positive or negative) related to purchases you've made for science projects from recommendations on our site, please let us know. Write to us at scibuddy@sciencebuddies.org.
Experimental Procedure
Warning: Hair dryers can cause burns and fires if used improperly. Ask an adult to help you with all parts of the procedure that involve using the hair dryer.
- First, you will test how long it takes the jumping disc to pop at room temperature. Note: The ambient temperature in a room can affect the results of this experiment, so it is important to do all of the trials within a short period of time. For example, do not do some of the trials in the middle of the day in a room that gets warmer when exposed to lots of sunlight, and then do the rest of the trials at night.
- Ask an adult to hold the jumping disc and use a hair dryer to heat it until it pops through to its "loaded" position (jumping discs are very light, so if you just put them down and aim the hair dryer at them, they will blow away). Be careful to aim the hair dryer away from the counter space where you will be testing the jumping disc — you do not want to heat this surface. Note: if you are working in a cold room and/or your hair dryer is not very powerful, the jumping disc may not pop on its own. In addition to heating it, you may need to give it a little push in the center of the disc. The disc will stay in the loaded position only if it is warm enough.
- Get your stopwatch ready. Have the adult put the jumping disc on the counter, with the "popped out" side facing up. As soon as he or she has put the jumping disc down, start your stopwatch.
- Watch closely! As soon as you see the jumping disc pop up from the counter, stop your stopwatch. Record this time in your lab notebook. You may want to create a table like Table 1.
- Repeat steps 1a to 1c to do another trial. To ensure that your results are accurate, do at least five trials total. (You can do more if you have time.)
| Time to jump (seconds) | |||
| Trial Number | Cold Counter | Room Temp. Counter | Hot Counter |
| 1 | |||
| 2 | |||
| 3 | |||
| 4 | |||
| 5 | |||
- Next, you will test how long it takes the disc to pop when the counter is hot.
- As you did in Step 1, ask an adult use the hair dryer to heat the jumping disc But this time, have the adult heat the counter first for about 30 seconds and then heat the jumping disc directly over the warm counter, so the counter continues to be heated.
- Get your stopwatch ready. Have the adult place the jumping disc down on the warm spot on the counter, with the popped-out side facing up. As soon as the jumping disc is on the counter, start your stopwatch.
- Watch closely! As soon as the disc pops up, stop your stopwatch and record the time.
- Repeat steps 2a to 2c until you have completed at least five trials total.
- Now you will test how long it takes the disc to pop when the counter is cold.
- Fill a plastic bag with ice, or use something else from your freezer as a substitute (anything frozen will be OK as long as it will make good contact with the surface of the counter — check with your parents first, as this may defrost the frozen item). Place this bag on a new spot on your counter — not the same spot from step 2, which might still be warm. Let the bag sit on the counter for at least one minute.
- Ask an adult to use the hair dryer to heat up the jumping disc until it pops. Keep the hair dryer away from your cold spot on the counter. Remember that you can give the disc a little push in the middle if it does not pop through on its own.
- Get your stopwatch ready. Move the bag of ice off of the counter, and quickly wipe off any water on the counter with a paper towel or dish towel. Have the adult place the jumping disc on the cold spot on the counter, and immediately start your stopwatch.
- Watch closely! As soon as the disc pops up, stop your stopwatch and record the time.
- Repeat steps 3a to 3d until you have done at least five trials total (you can re-use the same bag of ice as long as it has not melted completely.)
- Analyze your data.
- If you made a data table similar to Table 1, can you now use that data to make a graph? Figure 6 shows a blank example graph that you could fill in with your data.
- How does the temperature of the counter appear to change how long it takes the jumping disc to pop? Is the difference in jump time between "cold" and "room temperature" the same as the difference in time between "hot" and "room temperature"?
- What did you predict would happen? How do your results compare to your predictions?
 Image Credit: Ben Finio, Science Buddies / Science Buddies
Image Credit: Ben Finio, Science Buddies / Science Buddies
Figure 6. A blank example graph to plot your data, showing jump times versus counter temperature.
Ask an Expert
Global Connections
The United Nations Sustainable Development Goals (UNSDGs) are a blueprint to achieve a better and more sustainable future for all.
Variations
- In this project you examined results on a counter at only three temperatures — hot, cold, and room temperature. Can you be creative to figure out how you could test more temperatures? What about using different settings on your hair dryer to get different levels of "warm" and "hot," or something from your refrigerator instead of your freezer to get "cool" instead of "cold"? Can you use a thermometer to measure the temperatures exactly?
- You did all of your tests for this project on the same surface. However, different materials transfer heat at different rates. What happens if you repeat the tests on different surfaces like metal, wood, or tile? Does this change your results?
- Can you find other ways to make your disc click into the loaded position besides using a hair dryer? For example, what if you placed it on a metal object in direct sunlight? Warning: Make sure you have an adult help you with any experiments involving hot objects, so you do not burn yourself.
Careers
If you like this project, you might enjoy exploring these related careers:












