Summary
Introduction
You have probably cracked open a soda before to see the liquid fizz right up out of the bottle, creating a huge mess. Why does that happen? It has to do with the carbon dioxide gas that is added to the liquid to make it fizzy. Opening the bottle releases the built-up pressure by the gas inside, causing the gas-liquid mixture to rush out the bottle. In this activity, you will demonstrate with the help of air- and water-filled balloons how a gas changes volume depending on its pressure.
Materials
- Small balloons such as water balloons (2, additional 2 optional)
- 60 mL syringe (without needle) Note: the syringe needs to be airtight.
- Scissor
- Tap water
- Food color (optional)
 Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Prep Work
- Fill the syringe with water. Then fill one balloon with some of the water and tie its opening with a knot. Cut the neck off right above the knot. The balloon should still be small enough to fit into the syringe.
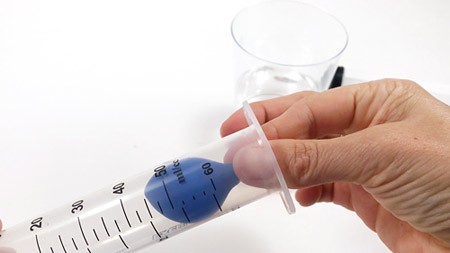 Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Image Credit: Svenja Lohner, Science Buddies / Science Buddies
- Use the syringe to fill the second balloon with a little bit of air. It should be the same size as the water-filled balloon. Again, tie the balloon opening with a knot and cut off the remaining parts right above the knot.
 Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Instructions
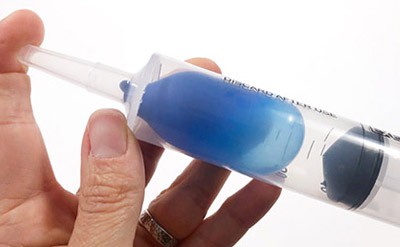
- Put the air-filled balloon inside the syringe at the very end. Insert the plunger into the syringe and try to push the balloon into the tip of the syringe.How hard is it to push the plunger in? What happens to the air inside the syringe?
- Pull the plunger back again and move the balloon into the middle of the syringe. Then close the front opening (the tip) of the syringe with one finger and push the plunger into the syringe again.What do you notice? How does the balloon look or change when you push the plunger in?
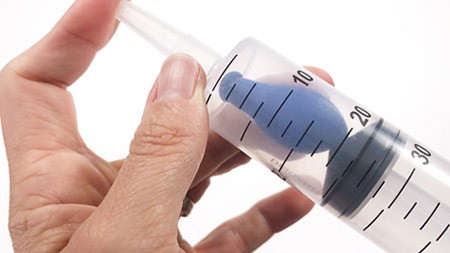 Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Image Credit: Svenja Lohner, Science Buddies / Science Buddies
- Release your finger from the tip of the syringe. Place the balloon into the tip of the syringe and push the plunger into the syringe until it touches the balloon. Then pull the plunger all the way back while again closing the tip of the syringe with your finger.Does the balloon shape change? How? Can you explain why?
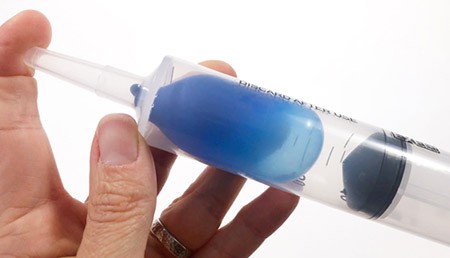 Image Credit: Svenja Lohner, Science Buddies / Science Buddies
Image Credit: Svenja Lohner, Science Buddies / Science Buddies
- Replace the air-filled balloon inside the syringe with the water-filled balloon. Then place the plunger into the syringe. Close the tip of the syringe with your finger and push the plunger into the syringe as far as you can.How does the balloon change this time?
- Release your finger from the tip of the syringe and push the plunger all the way into the syringe until it touches the balloon at the tip of the syringe. Then close the tip of the syringe again with your finger and try to pull the plunger back as far as you can.What happens to the water-filled balloon? Does it behave differently than the air-filled balloon? If yes, how and why?
Cleanup
What Happened?
Did you see the air inside the air-filled balloon contract and expand? You should have! Without closing the tip of the syringe with your finger, you can easily push on the plunger. The air can escape through the opening at the tip of the syringe. However, when you close the syringe with your finger, the air can't escape anymore. If you press on the plunger, you increase the pressure of the air and thus the air in the balloon contracts or decreases its volume. You should have seen the air-filled balloon shrivel up and get smaller in size. The opposite happens when you pull the plunger back while closing the opening of the syringe. This time, you decrease the pressure of the air inside the syringe and its volume increases. As a result, the air-filled balloon expands and grows in size. A perfect demonstration of Boyle's law!
The results look different with the water-filled balloon. Although you are compressing the air inside the syringe when pressing on the plunger, the water inside the balloon does not get compressed. The balloon stays the same size. The water balloon also keeps its shape when pulling out the plunger while closing the tip of the syringe. In contrast to gases, liquids are not compressible as their particles are already very close together. Boyle's law is only valid for gases.
When you filled the syringe with water as well, you still should have seen the air-filled balloon shrinking while pushing the plunger into the syringe. The air-filled balloon also should have expanded when pulling the plunger out while the tip of the syringe was closed. You might have noticed, though, that you were not able to push and pull the plunger in and out as far as you could with the air-filled syringe. This is again due to the fact that liquids cannot be compressed like gases. You should have observed that also when trying to push the plunger in or pulling it back in the water-filled syringe with the water-filled balloon. It was impossible to move the plunger in and out!
Digging Deeper
The difference between solids, liquids, and gases is how the particles (molecules or atoms) they are made of behave. Particles in solids are usually tightly packed in a regular pattern. Although the particles in a liquid are also close together, they are able to move freely with respect to one another. Gas particles, however, are widely spread out and occupy lots of space. They continue to spread to any space that is available. This means that in contrast to liquids and solids, the volume of a gas is not fixed. Robert Boyle, a chemist and physicist form the 17th century, discovered that the volume of gas, meaning how much space it occupies, is related to its pressure, and vice versa. He found that if you pressurize a gas at a constant temperature, its volume contracts. If you decrease its pressure, its volume increases.
You can observe a real-life application of Boyle's Law when you fill your bike tires with air. When you pump air into a tire, the gas molecules inside the tire get compressed and packed closer together. This increases the pressure of the gas, and it starts to push against the walls of the tire. You can feel how the tire becomes pressurized and tighter. Another example is the soda bottle. To get carbon dioxide gas into the liquid, the whole bottle is usually pressurized with gas. As long as the bottle is closed, it is very hard to squeeze, as the gas is confined to a small space and pushes against the bottle's walls. However, when you remove the cap, the available volume increases and the gas escapes. At the same time, its pressure decreases. If the gas/liquid mixture was shaken too much, the liquid would shoot out of the bottle together with the gas—and there you have the mess!
One rather important demonstration of Boyle's law is our own breathing. Inhaling and exhaling basically means increasing or decreasing the volume of our chest cavity. This creates low pressure or high pressure in our lungs, resulting in air either getting sucked into our lungs or leaving our lungs.
Ask an Expert
For Further Exploration
- Use the same setup as in your experiment, but this time, add water to your syringe in addition to the air-filled and water-filled balloons. You can add a drop of food coloring to make the water more visible. Then close the tip of the syringe and try to press the plunger into the syringe and pull it out again. What happens this time? How does the water inside the syringe make a difference?
Related Resources
Project Ideas
Links
- Blog Post: Gas and the Size of a Marshmallow