Abstract
Have you ever wondered how a chick breathes inside its shell? Every animal needs oxygen to survive, so the chick must get air somehow! Try this science project to discover the answer.Summary
Haleh Khoshnevisan, MedImmune
Sandra Slutz, PhD, Science Buddies
Teisha Rowland, PhD, Science Buddies
Objective
Determine if the pores in a chicken egg shell allow water to enter by soaking raw eggs in dye.
Introduction
Every animal requires oxygen to live. When animals, including humans, breathe in, oxygen enters the lungs, where it is shuttled into the blood stream and distributed to all the different parts of the body. The oxygen is used in an internal chemical reaction called metabolism to provide the animal with energy. The process of metabolism also produces a waste gas called carbon dioxide. In order to get rid of this waste gas, the blood stream carries the carbon dioxide back to the lungs where it is collected and finally breathed out.
Animals that grow inside their mothers, like humans, get their oxygen from their mothers. The blood stream of the baby animal and the mother are connected through an umbilical cord, which allows the baby to collect oxygen that his or her mother breathes in and use the mother's lungs to get rid of the carbon dioxide. But how do animals that grow in a shell and do not have umbilical cords, like chickens, take in oxygen and get rid of carbon dioxide?
Bird and reptile eggs have a hard shell. Directly under the shell are two membranes. When the eggs are laid by the mother, they are warmer than the air, and as they cool, the material inside the egg shrinks a little bit. This shrinking pulls the two membranes apart, leaving behind an air cell, also called an air sack, that is filled with oxygen. As the animal develops, it needs the oxygen replenished so it can continue to grow, and it needs the carbon dioxide it is making to be able to escape from the air cell. So, how does this happen? Well, if you examine a chicken egg carefully with a magnifying glass, you will see that there are tiny little holes, called pores, in the shell. A chicken egg shell has more than 7,000 pores! Do you think that the pores could be a way for carbon dioxide to escape and fresh air to get in? In this science project you will determine if substances can move in and out of an egg through these pores. To do this you will soak several raw chicken eggs in water with food color and detergent. Then you will crack the eggs open and look on the inside of their shells. If the pores really do allow materials to cross back and forth between the inside of the egg and the outside environment, then you should see dye on the inside of the shells. Ready to find out the answer? Time to dye some eggs!
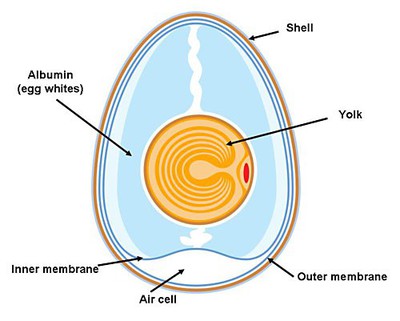
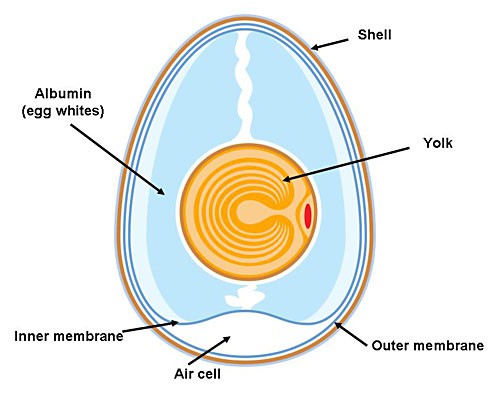 Image Credit: Wikipedia by Horst Frank and modified by Sandra Slutz / GNU Free Documentation License
Image Credit: Wikipedia by Horst Frank and modified by Sandra Slutz / GNU Free Documentation LicenseDiagram shows an egg is comprised of a shell and outer membrane that surrounds an interior filled with albumin (egg whites). At the bottom of the egg a small air pocket exists that separates the albumin from the outer membrance. A circular yolk stays suspended at the center of the albumin.
Terms and Concepts
- Oxygen
- Metabolism
- Carbon dioxide
- Umbilical cord
- Shell
- Air cell, also called an air sack
- Pore
Questions
- Why do animals need oxygen?
- Why do animals need to get rid of the carbon dioxide that their bodies make?
- What are the different parts of a chicken egg?
Bibliography
These websites have more information about breathing, oxygen, carbon dioxide, and metabolism.
- The Franklin Institute Science Museum. (2008). Respiratory System: Oxygen Delivery System. Retrieved May 22, 2008.
- Inselman, L. (2006, August). Your Lungs & Respiratory System. Retrieved May 22, 2008.
- Ophardt, C. (2003). Carbon Cycle. Retrieved May 22, 2008.
This website has more information about chicken eggs.
- 4-H Virtual Farm. (n.d.). The Parts of the Egg . Retrieved July 5, 2012.
Materials and Equipment
- Measuring cup
- Water
- Large pot or bowl
- Measuring teaspoon
- Liquid dishwashing detergent
- Blue food color
- Store-bought raw eggs (6)
- Tongs or large spoon
- Cup
- Plate or paper towel
- Optional: Camera
- Lab notebook
Experimental Procedure
- Pour 1.5 cups of water into a large pot or bowl.
-
Add ¼ teaspoon (tsp) of liquid dishwashing detergent and ¼ tsp of blue food color to the water in the bowl. Mix well.
- Make sure to get all of the detergent out of the measuring teaspoon and mixed into the bowl.
- Note: If you would like to know why you are using detergent in this experiment, and how it might affect your results, see the Technical Note, below.
-
Carefully set all five raw eggs in the pot.
- Make sure that all of the eggs are submerged in the liquid. If part of any egg is above the surface of the liquid, repeat steps 1-2 and add the extra liquid to the pot until the eggs are submerged. Use a larger pot to do this, if needed.
- In your lab notebook, write what time it is. You will be soaking the eggs for 24 hours.
- After the eggs have soaked in the liquid for 24 hours, carefully lift one of them out using the tongs or large spoon. How does the egg look?
-
Crack the raw egg into a cup, being careful not to damage or crush the shell much.
- You can later dispose of the raw egg (not the shell) by pouring it down the drain. (The eggs should not be eaten because they were soaked with dishwashing detergent.)
-
Set the empty egg's shell on a plate or paper towel and carefully inspect the inside of the shell.
- What do you see on the inside of the shell? Do you see any blue coloration? If so, does the coloration form any specific shapes that might be related to the egg's pores? Are there multiple places with blue coloration? Record your observations in your lab notebook.
- There may be blue lines where you cracked the shell open, and these lines can be ignored.
-
If you have a camera, you can take pictures of the inside of the shells.
- If your camera has a macro mode for taking pictures of small objects up-close, take pictures using that mode.
- You can print your pictures and put them in your lab notebook and on your Science Fair Project Display Board.
-
Repeat steps 5-8 until you have cracked open and examined the inside shell of each egg.
- Do not forget to record your observations in your lab notebook for each egg.
- Did the inside of all of the eggs' shells look the same, or were there differences? Can you explain why the inside of the shells looked like they did? Based on your data, do you think it is likely that oxygen and carbon dioxide can travel through the egg shell?
- When you are done making observations, thoroughly clean any surface the raw eggs (including the shells) touched because they may carry Salmonella. Also, wash your hands thoroughly with soap.
In this science project, the role of the detergent is to help break through the membranes of the egg so that the dye can make a concentrated, visible mark on the inside of the eggshell, rather than a light smear all over it. If you look at Figure 1 in the Background section, you can see that chicken eggs have a shell, followed by an outer membrane, and then an inner membrane. If there is a large enough pore/hole in the shell, the dye will get in; but without detergent to break through the membranes, the dye may get trapped and spread out between the shell and outer membrane, or between the two membranes, and not make a clear mark on the eggshell. The detergent does not affect the eggshell.
Ask an Expert
Global Connections
The United Nations Sustainable Development Goals (UNSDGs) are a blueprint to achieve a better and more sustainable future for all.
Variations
- Do fresh eggs and aged eggs behave similarly? Buy a dozen eggs whose expiration date is at least two weeks away. Try this experiment with half of the eggs right away. Let the other six eggs age in the refrigerator for two weeks. Repeat the experiment with the aged eggs. How does the data compare between the fresh and the aged eggs?
- Do other bird eggs give you similar results? Try it and find out! You can often find duck and quail eggs at Asian grocery stores.
- If the pores in the shells of chicken eggs really do allow materials to cross back and forth between the inside of the egg and the outside environment, then when eggs are boiled in water the air inside the egg should be replaced by water, and water is heavier than air. You can test this by labeling and weighing several raw eggs, cooking them until they are "hard boiled," and then weighing them again. You will need to use a scale that can distinguish changes as small as 0.1 grams, such as a triple-beam balance (which your school may have) or a very accurate electronic kitchen scale. Did each egg gain weight?
- For more interesting egg-based science fair projects, try Egg Substitutes.
Careers
If you like this project, you might enjoy exploring these related careers:











